Clindamycin Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- CLINDAMYCIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- MICROBIOLOGY
- INDICATIONS & USAGE
- CLINDAMYCIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- CLINDAMYCIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- REFERENCES
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
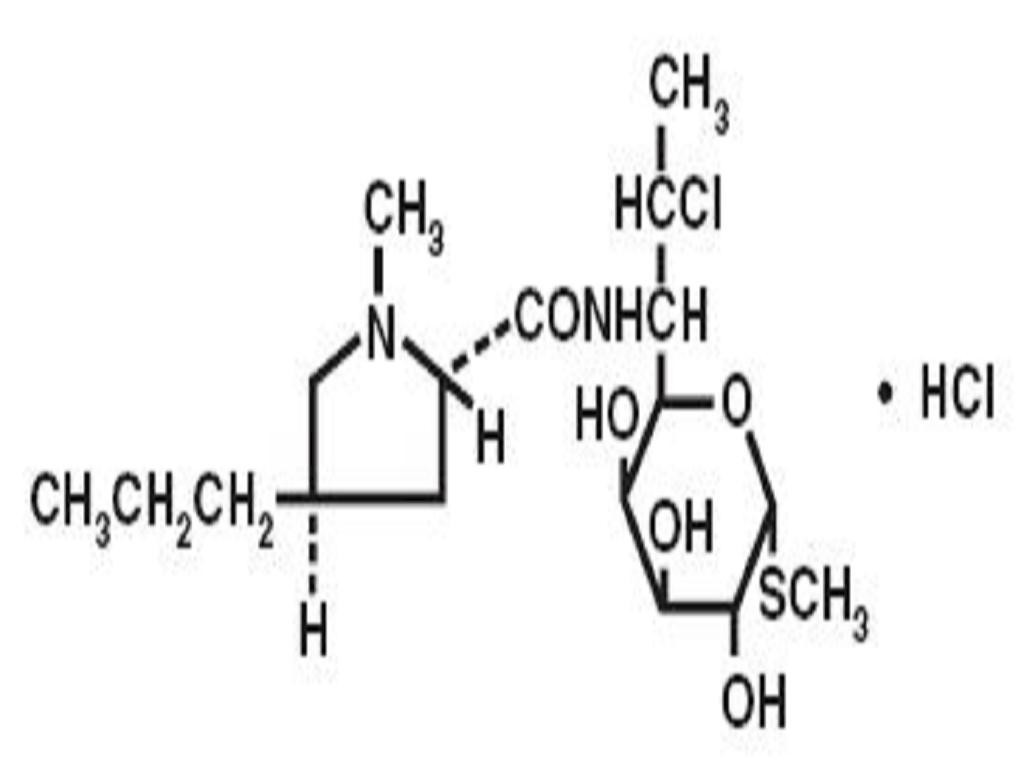
CLINDAMYCIN HYDROCHLORIDE DESCRIPTION
DESCRIPTION
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGYHuman Pharmacology
MICROBIOLOGY
MicrobiologySUSCEPTIBILITY TESTING METHODS
Dilution Techniques
Diffusion Techniques
Table 1. Susceptibility Interpretive Criteria for Clindamycin
SusceptibilityInterpretiveCriteriaMinimalDiskInhibitoryDiffusionPathogenConcentrations(Zone(MIC inDiametersmcg/mL)in mm)
Quality Control
Table 2. Acceptable Quality Control Ranges for Clindamycin to be Used in Validation of Susceptibility Test Results
AcceptableQualityControl RangesQC StrainMinimumDiskInhibitoryDiffusionConcentrations(Zone(MIC in Diametersmcg/mL)in mm)
INDICATIONS & USAGE
INDICATIONS AND USAGECLINDAMYCIN HYDROCHLORIDE CONTRAINDICATIONS
CONTRAINDICATIONSWARNINGS
WARNINGSPRECAUTIONS
PRECAUTIONSGeneral
INFORMATION FOR PATIENTS
Information for PatientsLABORATORY TESTS
Laboratory TestsDRUG INTERACTIONS
Drug InteractionsCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of FertilityPREGNANCY
Teratogenic effectsPregnancy category B
NURSING MOTHERS
Nursing MothersPEDIATRIC USE
Pediatric UseGERIATRIC USE
Geriatric UseCLINDAMYCIN HYDROCHLORIDE ADVERSE REACTIONS
ADVERSE REACTIONSOVERDOSAGE
OVERDOSAGEDOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATIONHOW SUPPLIED
STORAGE AND HANDLING
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
ANIMAL TOXICOLOGYREFERENCES
INACTIVE INGREDIENT
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Clindamycin HydrochlorideClindamycin Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!