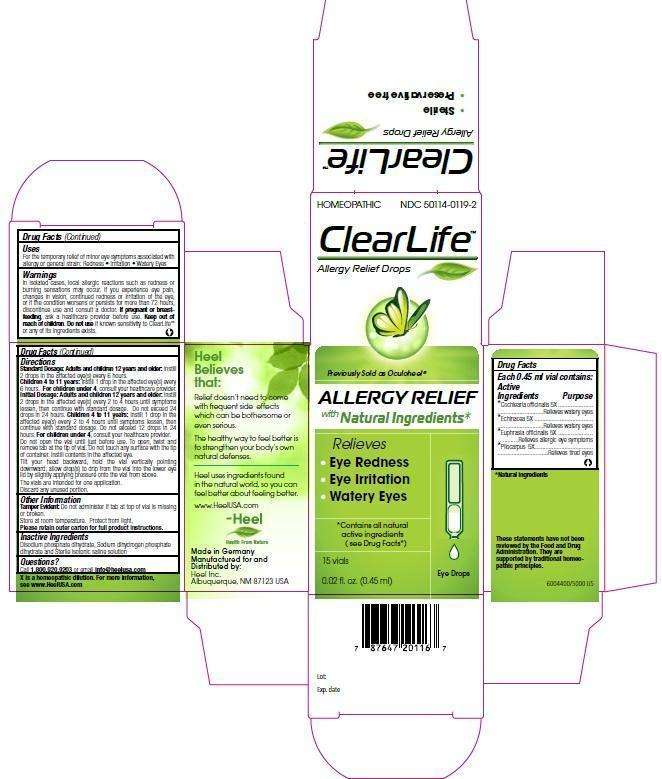
Clearlife
Clearlife Eye Drops
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- CLEARLIFE INDICATIONS AND USAGE
- WARNINGS
- CLEARLIFE DOSAGE AND ADMINISTRATION
- INACTIVE INGREDIENTS
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS
Each 0.45 ml vial contains: Cochlearia officinalis 5X, Echinacea 5X, Euphrasia officinalis 5X, Pilocarpus 5X
PURPOSE
Cochlearia officinalis 5X......Relieves watery eyes
Echinacea 5X......................Relieves watery eyes
Euphrasia officinalis 5X......Relieves allergic eye symptoms
Pilocarpus 5X.....................Relieves tired eyes
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children.
CLEARLIFE INDICATIONS AND USAGE
For the temporary relief of minor eye symptoms associated with allergy or general strain:
- Redness
- Irritation
- Watery Eyes
WARNINGS
In isolated cases, local allergic reactions such as redness or burning sensations may occur. If you experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours, discontinue use and consult a doctor. If pregnant or breast-feeding, ask a healthcare provider before use. Keep out of reach of children. Do not use if known sensitivity to ClearLife™ or any of its ingredients exists.
CLEARLIFE DOSAGE AND ADMINISTRATION
Standard Dosage:
Adults and children 12 years and older: Instill 2 drops in the affected eye(s) every 6 hours.
Children 4 to 11 years: Instill 1 drop in the affected eye(s) every 6 hours.
For children under 4, consult your healthcare provider.
Initial Dosage:
Adults and children 12 years and older: Instill 2 drops in the affected eye(s) every 2 to 4 hours until symptoms lessen, then continus with standard dosage. Do not exceed 24 drops in 24 hours.
Children 4 to 11 years: Instill 1 drop in the affected eye(s) every 2 to 4 hours until symptoms lessen, then continus with standard dosage. Do not exceed 12 drops in 24 hours.
For children under 4, consult your healthcare provider.
Do not open the vial until just before use. To open, twist and remove tab at the top of vial. Do not touch any surface with the tip of the container. Instill contents in the affected eye.
Tilt your head backward, hold the vial vertically pointing downward; allow drop(s) to drip from the vial into the lower eye lid by slightly applying pressure onto the vial from above.
The vials are intended for one application. Discard any unused portion.
INACTIVE INGREDIENTS
Disodium phosphate dihydrate, Sodium dihydrogen phosphate dihydrate and Sterile isotonic saline solution.
ClearlifeCOCHLEARIA OFFICINALIS FLOWERING TOP, ECHINACEA, UNSPECIFIED, EUPHRASIA STRICTA and PILOCARPUS JABORANDI LEAF SOLUTION/ DROPS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||