SHISEIDO CO., LTD.
Cle de peau Beaute Powder Foundation
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
|
Active ingredients
|
Purpose
|
| OCTINOXATE 1.9% |
Sunscreen |
| TITANIUM DIOAXIDE 15.0% |
Sunscreen |
CLE DE PEAU BEAUTE Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
Warnings
For external use only
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash or irritation develops and lasts.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply evenly before sun exposure and as needed
- children under 6 months of age: ask a doctor
Inactive ingredients
TALC, SYNTHETIC FLUORPHLOGOPITE, BARIUM SULFATE, DIMETHICONE, TRIETHYLHEXANOIN, BIS-BEHENYL/PHYTOSTERYL DIMER DILINOLEATE, VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER, SILICA, NYLON-12, SILICA DIMETHYL SILYLATE, TOCOPHERYL ACETATE, PRUNUS SPECIOSA LEAF EXTRACT, SODIUM ACETYLATED HYALURONATE, ROSA ROXBURGHII FRUIT EXTRACT CAMELLIA SINENSIS LEAF EXTRACT, SORBITAN SESQUIISOSTEARATE, TRIETHOXYSILYLETHYL POLYDIMETHYLSILOXYETHYL HEXYL DIMETHICONE, METHICONE, ALUMINUM HYDROXIDE, ALUMINUM DISTEARATE, TRIETHOXYSILYLETHYL POLYDIMETHYLSILOXYETHYL DIMETHICONE, SODIUM MAGNESIUM SILICATE, POLYSILICONE-2, GLYCERIN, TOCOPHEROL, WATER, BUTYLENE GLYCOL, ALCOHOL, BHT, CHLORPHENESIN, FRAGRANCE, MICA, ZINC OXIDE, IRON OXIDES
Questions ?
1-800-906-7503
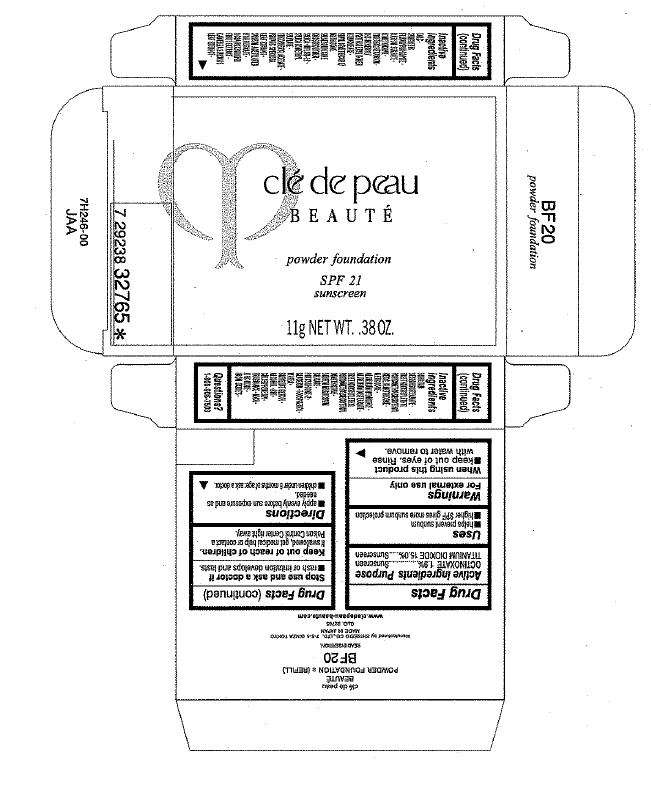
PRINCIPAL DISPLAY PANEL - 11g Carton
clé de peau
BEAUTÉ
powder foundation
SPF 21
sunscreen
11g NET WT. .38 OZ.
CLE DE PEAU BEAUTE
Octinoxate and Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-300 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
11 in 1 TRAY |
|
|
|
2 |
NDC:52685-300-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
CLE DE PEAU BEAUTE
Octinoxate and Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-301 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
11 in 1 TRAY |
|
|
|
2 |
NDC:52685-301-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
CLE DE PEAU BEAUTE
Octinoxate and Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-302 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
11 in 1 TRAY |
|
|
|
2 |
NDC:52685-302-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
CLE DE PEAU BEAUTE
Octinoxate and Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-303 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
11 in 1 TRAY |
|
|
|
2 |
NDC:52685-303-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
CLE DE PEAU BEAUTE
Octinoxate and Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-304 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
11 in 1 TRAY |
|
|
|
2 |
NDC:52685-304-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
CLE DE PEAU BEAUTE
Octinoxate and Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-305 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
11 in 1 TRAY |
|
|
|
2 |
NDC:52685-305-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
CLE DE PEAU BEAUTE
Octinoxate and Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-306 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
11 in 1 TRAY |
|
|
|
2 |
NDC:52685-306-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
CLE DE PEAU BEAUTE
Octinoxate and Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-307 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
11 in 1 TRAY |
|
|
|
2 |
NDC:52685-307-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
