Clarithromycin
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
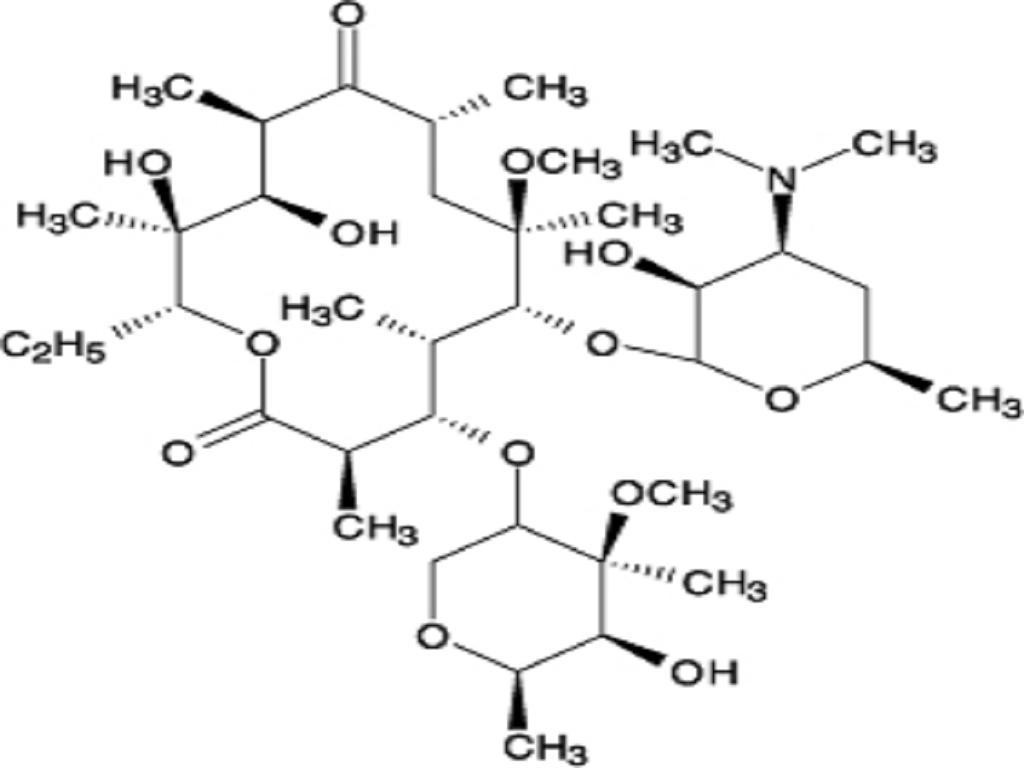
- CLARITHROMYCIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- MICROBIOLOGY
- INDICATIONS & USAGE
- CLARITHROMYCIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- CLARITHROMYCIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- CLINICAL STUDIES
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
CLARITHROMYCIN DESCRIPTION
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
PRECAUTIONSDOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGY
MICROBIOLOGY
INDICATIONS AND USAGE
Aerobic Gram-positive Microorganisms
Aerobic Gram-negative Microorganisms
Other Microorganisms
Mycobacteria
INDICATIONS AND USAGE
Helicobacter
Pretreatment Resistance
*
*
Amoxicillin Susceptibility Test Results and Clinical/Bacteriological Outcomes
Aerobic Gram-positive Microorganisms
Aerobic Gram-negative Microorganisms
Anaerobic Gram-positive Microorganisms
Anaerobic Gram-negative Microorganisms
Susceptibility Testing Excluding Mycobacteria and Helicobacter
Dilution Techniques
*
*
*
*
**
Diffusion Techniques
*
*
*
*
**
In vitro Activity of Clarithromycin against Mycobacteria
Susceptibility Testing for Mycobacterium avium Complex (MAC)
Susceptibility Test for Helicobacter pylori
***
**
INDICATIONS & USAGE
Adults
Microbiology
Children
CLINICAL STUDIES: Otitis Media
Prophylaxis
CLARITHROMYCIN CONTRAINDICATIONS
Drug Interactions
CONTRAINDICATIONS
WARNINGS
PRECAUTIONS: PregnancyPRECAUTIONS
WARNINGS
PRECAUTIONS
GeneralDOSAGE AND ADMINISTRATION
PRECAUTIONS
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
CONTRAINDICATIONS
WARNINGS
Antiarrhythmics
Ergotamine/Dihydroergotamine
CONTRAINDICATIONS
Triazolobenziodidiazepines (such as Triazolam and Alprazolam) and Related Benzodiazepines (such as Midazolam)
HMG-CoA Reductase Inhibitors
Sildenafil (Viagra)
CONTRAINDICATIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category C
WARNINGS
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
WARNINGSPRECAUTIONSCLARITHROMYCIN ADVERSE REACTIONS
Post-Marketing Experience
WARNINGSPRECAUTIONS
Changes in Laboratory Values
OVERDOSAGE
DOSAGE & ADMINISTRATION
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
Triple Therapy: Clarithromycin/Lansoprazole/Amoxicillin
INDICATIONS AND USAGECLINICAL STUDIES
Triple Therapy: Clarithromycin/Omeprazole/Amoxicillin
INDICATIONS AND USAGECLINICAL STUDIES
Dual Therapy: Clarithromycin/Omeprazole
INDICATIONS AND USAGECLINICAL STUDIES
Dual Therapy: Clarithromycin/Ranitidine Bismuth Citrate
INDICATIONS AND USAGECLINICAL STUDIES
Children
Mycobacterial Infections
Prophylaxis
Treatment
CLINICAL STUDIES
HOW SUPPLIED
CLINICAL STUDIES
Mycobacterial InfectionsProphylaxis
MAC Bacteremia
Survival
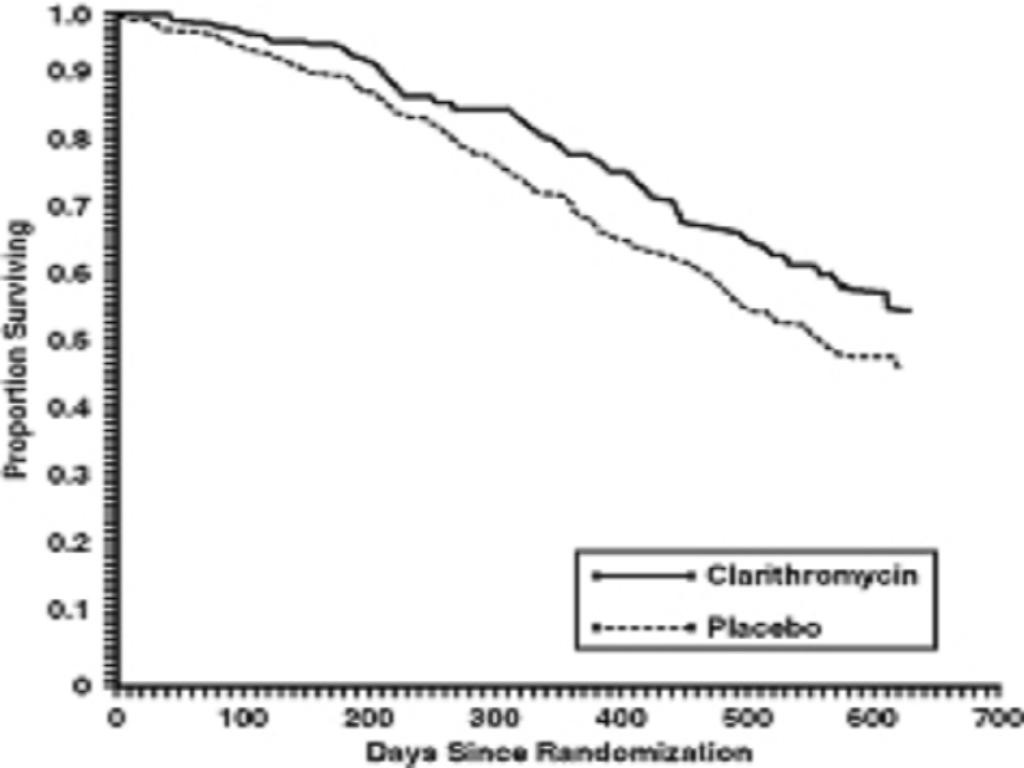
Clinically Significant Disseminated MAC Disease
Safety
*
*
Changes in Laboratory Values of Potential Clinical Importance
*
*
Treatment
MAC Bacteremia
Clinically Significant Disseminated MAC Disease
Survival
Safety
*
*
Changes in Laboratory Values
*
*
Otitis Media
*PathogenOutcomeS. pneumoniaeclarithromycin success rate, 13/15 (87%), control 4/5H. influenzae*
Safety
**
Safety
Duodenal Ulcer Associated with H. pylori Infection
Clarithromycin + Lansoprazole and Amoxicillin
H. pylori Eradication for Reducing the Risk of Duodenal Ulcer Recurrence
Triple therapy: Clarithromycin 500 mg b.i.d. + Lansoprazole 30 mg b.i.d. + Amoxicillin 1 gm b.i.d.
**
Clarithromycin + Omeprazole and Amoxicillin Therapy
H. pylori Eradication for Reducing the Risk of Duodenal Ulcer Recurrence
*histology, and/or culture. Patients were included in the analysis if they completed the study. Additionally, if patients dropped out of the study due to an adverse event related to the study drug, they were included in the analysis as failures of therapy. The impact of eradication on ulcer recurrence has not been assessed in patients with a past history of ulcer.**
Safety
ADVERSE REACTIONS
Clarithromycin + Omeprazole Therapy
Duodenal Ulcer Healing
***
Eradication of H. pylori Associated with Duodenal Ulcer
***
**
Safety
**
Changes in Laboratory Values
ADVERSE REACTIONS
Clarithromycin + Ranitidine Bismuth Citrate Therapy
Safety
ADVERSE REACTIONS
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

ClarithromycinClarithromycin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!