Clarithromycin
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
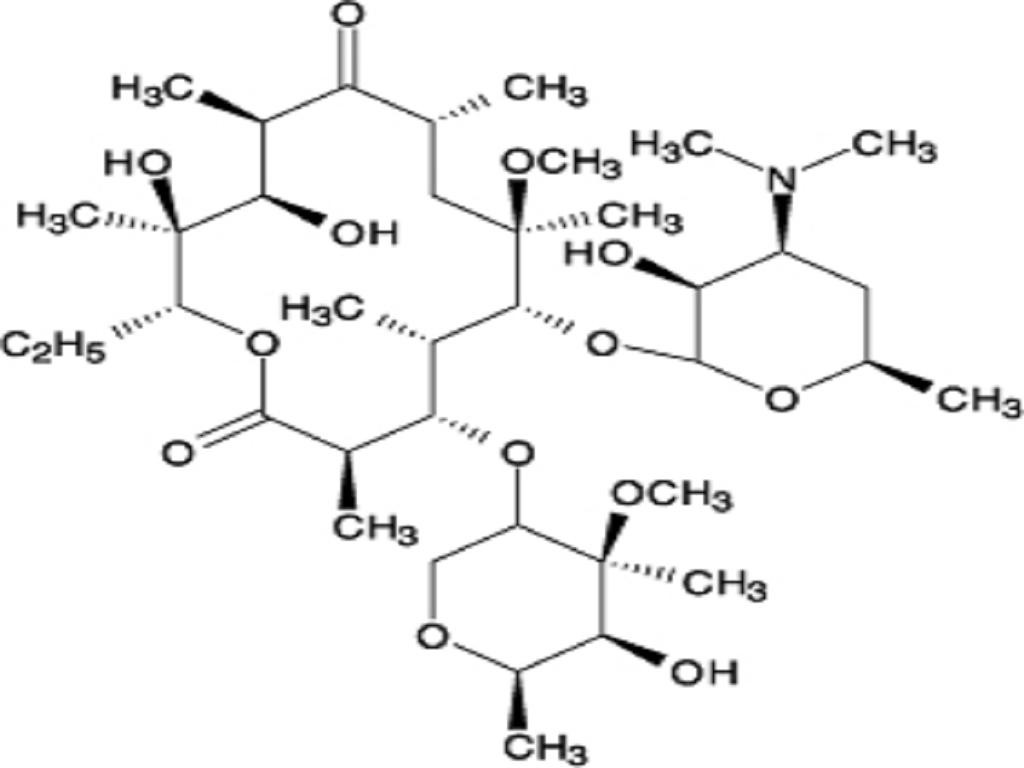
- CLARITHROMYCIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- MICROBIOLOGY
- INDICATIONS & USAGE
- CLARITHROMYCIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- CLARITHROMYCIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- CLINICAL STUDIES
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
CLARITHROMYCIN DESCRIPTION
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
PRECAUTIONSDOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGY
MICROBIOLOGY
INDICATIONS AND USAGE
Aerobic Gram-positive Microorganisms
Aerobic Gram-negative Microorganisms
Other Microorganisms
Mycobacteria
INDICATIONS AND USAGE
Helicobacter
Pretreatment Resistance
*
*
Amoxicillin Susceptibility Test Results and Clinical/Bacteriological Outcomes
Aerobic Gram-positive Microorganisms
Aerobic Gram-negative Microorganisms
Anaerobic Gram-positive Microorganisms
Anaerobic Gram-negative Microorganisms
Susceptibility Testing Excluding Mycobacteria and Helicobacter
Dilution Techniques
*
*
*
*
**
Diffusion Techniques
*
*
*
*
**
In vitro Activity of Clarithromycin against Mycobacteria
Susceptibility Testing for Mycobacterium avium Complex (MAC)
Susceptibility Test for Helicobacter pylori
***
**
INDICATIONS & USAGE
Adults
Microbiology
Children
CLINICAL STUDIES: Otitis Media
Prophylaxis
CLARITHROMYCIN CONTRAINDICATIONS
Drug Interactions
CONTRAINDICATIONS
WARNINGS
PRECAUTIONS: PregnancyPRECAUTIONS
WARNINGS
PRECAUTIONS
GeneralDOSAGE AND ADMINISTRATION
PRECAUTIONS
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
CONTRAINDICATIONS
WARNINGS
Antiarrhythmics
Ergotamine/Dihydroergotamine
CONTRAINDICATIONS
Triazolobenziodidiazepines (such as Triazolam and Alprazolam) and Related Benzodiazepines (such as Midazolam)
HMG-CoA Reductase Inhibitors
Sildenafil (Viagra)
CONTRAINDICATIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category C
WARNINGS
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
WARNINGSPRECAUTIONSCLARITHROMYCIN ADVERSE REACTIONS
Post-Marketing Experience
WARNINGSPRECAUTIONS
Changes in Laboratory Values
OVERDOSAGE
DOSAGE & ADMINISTRATION
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
Triple Therapy: Clarithromycin/Lansoprazole/Amoxicillin
INDICATIONS AND USAGECLINICAL STUDIES
Triple Therapy: Clarithromycin/Omeprazole/Amoxicillin
INDICATIONS AND USAGECLINICAL STUDIES
Dual Therapy: Clarithromycin/Omeprazole
INDICATIONS AND USAGECLINICAL STUDIES
Dual Therapy: Clarithromycin/Ranitidine Bismuth Citrate
INDICATIONS AND USAGECLINICAL STUDIES
Children
Mycobacterial Infections
Prophylaxis
Treatment
CLINICAL STUDIES
HOW SUPPLIED
CLINICAL STUDIES
Mycobacterial InfectionsProphylaxis
MAC Bacteremia
Survival
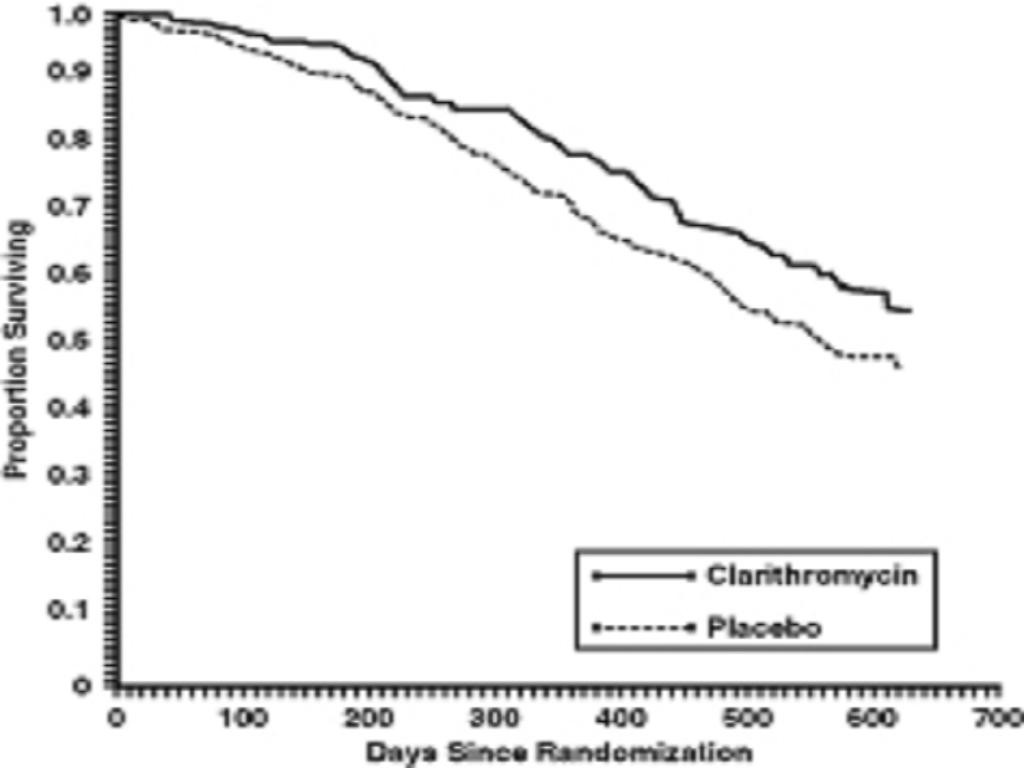
Clinically Significant Disseminated MAC Disease
Safety
*
*
Changes in Laboratory Values of Potential Clinical Importance
*
*
Treatment
MAC Bacteremia
Clinically Significant Disseminated MAC Disease
Survival
Safety
*
*
Changes in Laboratory Values
*
*
Otitis Media
*PathogenOutcomeS. pneumoniaeclarithromycin success rate, 13/15 (87%), control 4/5H. influenzae*clarithromycin success rate, 10/14 (71%), control 3/4M. catarrhalisclarithromycin success rate, 4/5, control 1/1S. pyogenesclarithromycin success rate, 3/3, control 0/1Overallclarithromycin success rate, 30/37 (81%), control 8/11 (73%)
Safety
The incidence of adverse events in all patients treated, primarily diarrhea and vomiting, did not differ clinically or statistically for the two agents.
In two other controlled clinical trials of acute otitis media performed in the United States, where significant rates of beta-lactamase producing organisms were found, clarithromycin was compared to an oral antimicrobial agent that contained a specific beta-lactamase inhibitor. In these studies, very strict evaluability criteria were used to determine the clinical responses. In the 233 patients who were evaluated for clinical efficacy, the combined clinical success rate (i.e., cure and improvement) at the post-therapy visit was 91% for both clarithromycin and the control.
For the patients who had microbiologic determinations at the pre-treatment visit, the following presumptive bacterial eradication/clinical cure outcomes (i.e., clinical success) were obtained:
Two U.S. Acute Otitis Media Studies Clarithromycin vs. Antimicrobial/Beta-lactamase Inhibitor Efficacy Results
*Of the H. influenzae isolated pre-treatment, 3% were resistant to clarithromycin and 10% were resistant to the control agent.PathogenOutcomeS. pneumoniaeclarithromycin success rate, 43/51 (84%), control 55/56 (98%)H. influenzae*clarithromycin success rate, 36/45 (80%), control 31/33 (94%)M. catarrhalisclarithromycin success rate, 9/10 (90%), control 6/6S. pyogenesclarithromycin success rate, 3/3, control 5/5Overallclarithromycin success rate, 91/109 (83%), control 97/100 (97%)
Safety
The incidence of adverse events in all patients treated, primarily diarrhea (15% vs. 38%) and diaper rash (3% vs. 11%) in young children, was clinically and statistically lower in the clarithromycin arm versus the control arm.
Duodenal Ulcer Associated with H. pylori Infection
Clarithromycin + Lansoprazole and Amoxicillin
H. pylori Eradication for Reducing the Risk of Duodenal Ulcer Recurrence
Two U.S. randomized, double-blind clinical studies in patients with H. pylori and duodenal ulcer disease (defined as an active ulcer or history of an active ulcer within one year) evaluated the efficacy of clarithromycin in combination with lansoprazole and amoxicillin capsules as triple 14-day therapy for eradication of H. pylori. Based on the results of these studies, the safety and efficacy of the following eradication regimen were established:
Triple therapy: Clarithromycin 500 mg b.i.d. + Lansoprazole 30 mg b.i.d. + Amoxicillin 1 gm b.i.d.
Treatment was for 14 days. H. pylori eradication was defined as two negative tests (culture and histology) at 4 to 6 weeks following the end of treatment.
The combination of clarithromycin plus lansoprazole and amoxicillin as triple therapy was effective in eradicating H. pylori. Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence.
A randomized, double-blind clinical study performed in the U.S. in patients with H. pylori and duodenal ulcer disease (defined as an active ulcer or history of an ulcer within one year) compared the efficacy of clarithromycin in combination with lansoprazole and amoxicillin as triple therapy for 10 and 14 days. This study established that the 10-day triple therapy was equivalent to the 14-day triple therapy in eradicating H. pylori.
H. pylori Eradication Rates-Triple Therapy (Clarithromycin/Lansoprazole/Amoxicillin) Percent of Patients Cured [95% Confidence Interval] (number of patients)
*Based on evaluable patients with confirmed duodenal ulcer (active or within one year) and H. pylori infection at baseline defined as at least two of three positive endoscopic tests from CLOtest(Delta West LTD., Bentley, Australia), histology, and/or culture. Patients were included in the analysis if they completed the study. Additionally, if patients were dropped out of the study due to an adverse event related to the study drug, they were included in the analysis as evaluable failures of therapy.Patients were included in the analysis if they had documented H. pylori infection at baseline as defined above and had a confirmed duodenal ulcer (active or within one year). All dropouts were included as failures of therapy.(p<0.05) versus clarithromycin/lansoprazole and lansoprazole/amoxicillin dual therapy.(p<0.05) versus clarithromycin/amoxicillin dual therapy.The 95% confidence interval for the difference in eradication rates, 10-day minus 14-day, is (-10.5, 8.1) in the evaluable analysis and (-9.7, 9.1)in the intent-to-treat analysis.StudyDurationTriple Therapy Evaluable Analysis*Triple Therapy Intent-to-Treat AnalysisM93-13114 days92[80.0 - 97.7] (n = 48)86c [73.3 - 93.5] (n = 55)M95-39214 days86[75.7 - 93.6] (n = 66)83[72.0 - 90.8] (n = 70)M95-39914 days85 [77.0 - 91] (n = 113)82 [73.9 - 88.1] (n = 126)10 days84 [76.0 - 89.8] (n = 123)81 [73.9 - 87.6] (n = 135)
Clarithromycin + Omeprazole and Amoxicillin Therapy
H. pylori Eradication for Reducing the Risk of Duodenal Ulcer Recurrence
Three U.S., randomized, double-blind clinical studies in patients with H. pylori infection and duodenal ulcer disease (n = 558) compared clarithromycin plus omeprazole and amoxicillin to clarithromycin plus amoxicillin. Two studies (Studies 126 and 127) were conducted in patients with an active duodenal ulcer, and the third study (Study 446) was conducted in patients with a duodenal ulcer in the past 5 years, but without an ulcer present at the time of enrollment. The dosage regimen in the studies was clarithromycin 500 mg b.i.d. plus omeprazole 20 mg b.i.d. plus amoxicillin 1 gram b.i.d. for 10 days. In Studies 126 and 127, patients who took the omeprazole regimen also received an additional 18 days of omeprazole 20 mg q.d. Endpoints studied were eradication of H. pylori and duodenal ulcer healing (studies 126 and 127 only). H. pylori status was determined by CLOtesthistology, and culture in all three studies. For a given patient, H. pylori was considered eradicated if at least two of these tests were negative, and none was positive. The combination of clarithromycin plus omeprazole and amoxicillin was effective in eradicating H. pylori.
Per-Protocol and Intent-To-Treat H. pylori Eradication Rates % of Patients Cured [95% Confidence Interval]
*histology, and/or culture. Patients were included in the analysis if they completed the study. Additionally, if patients dropped out of the study due to an adverse event related to the study drug, they were included in the analysis as failures of therapy. The impact of eradication on ulcer recurrence has not been assessed in patients with a past history of ulcer.Patients were included in the analysis if they had documented H. pylori infection at baseline and had confirmed duodenal ulcer disease. All dropouts were included as failures of therapy.p<0.05 versus clarithromycin plus amoxicillin.Clarithromycin + Omeprazole + AmoxicillinClarithromycin + AmoxicillinPer- Protocol*Intent- To-TreatPer- Protocol*Intent- To-TreatStudy 12677 [64, 86] (n = 64)69 [57, 79] (n = 80)43 [31, 56] (n = 67)37 [27, 48] (n = 84)Study 12778 [67, 88] (n = 65)73 [61, 82] (n = 77)41 [29, 54] (n = 68)36 [26, 47] (n = 84)Study M96-44690 [80, 96] (n = 69)83 [74, 91] (n = 84)33 [24, 44] (n = 93)32 [23, 42] (n = 99)
Safety
In clinical trials using combination therapy with clarithromycin plus omeprazole and amoxicillin, no adverse reactions peculiar to the combination of these drugs have been observed. Adverse reactions that have occurred have been limited to those that have been previously reported with clarithromycin, omeprazole, or amoxicillin.
The most frequent adverse experiences observed in clinical trials using combination therapy with clarithromycin plus omeprazole and amoxicillin (n=274) were diarrhea (14%), taste perversion (10%), and headache (7%).
For information about adverse reactions with omeprazole or amoxicillin, refer to theADVERSE REACTIONSsection of their package inserts.
Clarithromycin + Omeprazole Therapy
Four randomized, double-blind, multi-center studies (067, 100, 812b, and 058) evaluated clarithromycin 500 mg t.i.d. plus omeprazole 40 mg q.d. for 14 days, followed by omeprazole 20 mg q.d. (067, 100, and 058) or by omeprazole 40 mg q.d. (812b) for an additional 14 days in patients with active duodenal ulcer associated with H. pylori. Studies 067 and 100 were conducted in the U.S. and Canada and enrolled 242 and 256 patients, respectively. H. pylori infection and duodenal ulcer were confirmed in 219 patients in Study 067 and 228 patients in Study 100. These studies compared the combination regimen to omeprazole and clarithromycin monotherapies. Studies 812b and 058 were conducted in Europe and enrolled 154 and 215 patients, respectively.
H. pylori infection and duodenal ulcer were confirmed in 148 patients in Study 812b and 208 patients in Study 058. These studies compared the combination regimen to omeprazole monotherapy. The results for the efficacy analyses for these studies are described below.
Duodenal Ulcer Healing
The combination of clarithromycin and omeprazole was as effective as omeprazole alone for healing duodenal ulcer.
End-of-Treatment Ulcer Healing Rates Percent of Patients Healed (n/N)
*p<0.05 for clarithromycin + omeprazole versus clarithromycin monotherapy.In Study 812b patients received omeprazole 40 mg daily for days 15 to 28.StudyClarithromycin + OmeprazoleOmeprazoleClarithromycinU.S. Studies Study 100 Study 06794% (58/62)*88% (56/64)*88% (60/68) 85% (55/65)71% (49/69) 64% (44/69)Non-U.S. Studies Study 058 Study 812b99% (84/85) 100% (64/64)95% (82/86) 99% (71/72)N/A N/A
Eradication of H. pylori Associated with Duodenal Ulcer
The combination of clarithromycin and omeprazole was effective in eradicating H. pylori.
H. pylori Eradication Rates (Per-Protocol Analysis) at 4 to 6 weeks Percent of Patients Cured (n/N)
*Statistically significantly higher than clarithromycin monotherapy (p<0.05)Statistically significantly higher than omeprazole monotherapy (p<0.05).StudyClarithromycin + OmeprazoleOmeprazoleClarithromycinU.S. Studies Study 100 Study 06764% (39/61)*74% (39/53)*0% (0/59) 0% (0/54)39% (17/44) 31% (13/42)Non-U.S. Studies Study 058 Study 812b74% (64/86)83% (50/60)1% (1/90) 1% (1/74)N/A N/AH. pylori eradication was defined as no positive test (culture or histology) at 4 weeks following the end of treatment, and two negative tests were required to be considered eradicated. In the per-protocol analysis, the following patients were excluded: dropouts, patients with major protocol violations, patients with missing H. pylori tests post-treatment, and patients that were not assessed for H. pylori eradication at 4 weeks after the end of treatment because they were found to have an unhealed ulcer at the end of treatment.
Ulcer recurrence at 6-months following the end of treatment was assessed for patients in whom ulcers were healed post-treatment.
Ulcer Recurrence at 6 months by H. pylori Status at 4 to 6 Weeks
*See 12-Month Recurrence Rates.H. pylori NegativeH. pylori PositiveU.S. Studies Study 100 Clarithromycin + Omeprazole Omeprazole Clarithromycin Study 067 Clarithromycin + Omeprazole Omeprazole Clarithromycin6% (2/34) - (0/0) 12% (2/17) 38% (11/29) - (0/0) 18% (2/11)56% (9/16) 71% (35/49) 32% (7/22) 50% (6/12) 67% (31/46) 52% (14/27)Non-U.S. Studies Study 058 Clarithromycin + Omeprazole Omeprazole Study 812b*Clarithromycin + Omeprazole Omeprazole6% (3/53) 0% (0/3) 5% (2/42) 0% (0/1)24% (4/17) 55% (39/71) 0% (0/7) 54% (32/59)12-Month Recurrence Rates Clarithromycin + Omeprazole Omeprazole3% (1/40) 0% (0/1)0% (0/6) 67% (29/43)Thus, in patients with duodenal ulcer associated with H. pylori infection, eradication of H. pylori reduced ulcer recurrence.
Safety
The adverse event profiles for the four studies showed that the combination of clarithromycin 500 mg t.i.d. and omeprazole 40 mg q.d. for 14 days, followed by omeprazole 20 mg q.d. (067, 100, and 058) or 40 mg q.d.(812b) for an additional 14 days was well tolerated. Of the 346 patients who received the combination, 12 (3.5%) patients discontinued study drug due to adverse events.
Adverse Events with an Incidence of 3% or Greater
*Studies 067 and 100, onlyAdverse EventClarithromycin + Omeprazole (n = 346) % of PatientsOmeprazole (n = 355) % of PatientsClarithromycin (n = 166) % of Patients*Taste Perversion15%1%16%Nausea5%1%3%Headache5%6%9%Diarrhea4%3%7%Vomiting4%<1%1%Abdominal Pain3%2%1%Infection3%4%2%Most of these events were mild to moderate in severity.
Changes in Laboratory Values
Changes in laboratory values with possible clinical significance in patients taking clarithromycin and omeprazole were as follows:
Hepatic
Renalelevated serum creatinine <1%.
For information on omeprazole, refer to theADVERSE REACTIONSsection of the PRILOSEC package insert.
Clarithromycin + Ranitidine Bismuth Citrate Therapy
In a U.S. double-blind, randomized, multicenter, dose-comparison trial, ranitidine bismuth citrate 400 mg b.i.d. for 4 weeks plus clarithromycin 500 mg b.i.d. for the first 2 weeks was found to have an equivalent H. pylori eradication rate (based on culture and histology) when compared to ranitidine bismuth citrate 400 mg b.i.d. for 4 weeks plus clarithromycin 500 mg t.i.d. for the first 2 weeks. The intent-to-treat H. pylori eradication rates are shown below:
H. pylori Eradication Rates in Study H2BA-3001
AnalysisRBC 400 mg + Clarithromycin 500 mg b.i.d.RBC 400 mg + Clarithromycin 500 mg t.i.d95% CI Rate DifferenceITT65% (122/188) [58%, 72%]63% (122/195) [55%, 69%](-8%, 12%)Per-Protocol72% (117/162) [65%, 79%]71% (120/170) [63%,77%](-9%, 12%)H. pylori eradication was defined as no positive test at 4 weeks following the end of treatment. Patients must have had two tests performed, and these must have been negative to be considered eradicated of H. pylori. The following patients were excluded from the per-protocol analysis: patients not infected with H. pylori prestudy, dropouts, patients with major protocol violations, patients with missing H. pylori tests. Patients excluded from the intent-to-treat analysis included those not infected with H. pylori prestudy and those with missing H. pylori tests prestudy.
Patients were assessed for H. pylori eradication (4 weeks following treatment) regardless of their healing status (at the end of treatment).
The relationship between H. pylori eradication and duodenal ulcer recurrence was assessed in a combined analysis of six U.S. randomized, double-blind, multicenter, placebo-controlled trials using ranitidine bismuth citrate with or without antibiotics. The results from approximately 650 U.S. patients showed that the risk of ulcer recurrence within 6 months of completing treatment was two times less likely in patients whose H. pylori infection was eradicated compared to patients in whom H. pylori infection was not eradicated.
Safety
In clinical trials using combination therapy with clarithromycin plus ranitidine bismuth citrate, no adverse reactions peculiar to the combination of these drugs (using clarithromycin twice daily or three times a day) were observed. Adverse reactions that have occurred have been limited to those reported with clarithromycin or ranitidine bismuth citrate. (SeeADVERSE REACTIONSsection of the Tritec package insert.) The most frequent adverse experiences observed in clinical trials using combination therapy with clarithromycin (500 mg three times a day) with ranitidine bismuth citrate (n = 329) were taste disturbance (11%), diarrhea (5%), nausea and vomiting (3%). The most frequent adverse experiences observed in clinical trials using combination therapy with clarithromycin (500 mg twice daily) with ranitidine bismuth citrate (n = 196) were taste disturbance (8%), nausea and vomiting (5%), and diarrhea (4%).
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

ClarithromycinClarithromycin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!