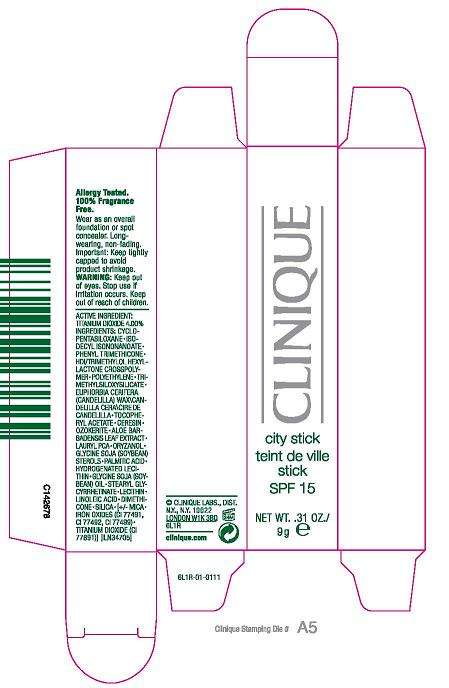
CITY STICK
FULL PRESCRIBING INFORMATION
Active ingredient
ACTIVE INGREDIENT: TITANIUM DIOXIDE, 4%
Uses
USAGE: KEEP TIGHTLY CAPPED TO AVOID PRODUCT SHRINKAGE
WARNING: KEEP OUT OF EYES. STOP USE IF IRRITATION OCCURS. KEEP OUT OF REACH OF CHILDREN
CLINIQUE CITY STICK SPF 15
0.31 OZ/ 9 g
CITY STICKTITANIUM DIOXIDE STICK
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!