citroma
Geiss, Destin & Dunn, Inc.
Vi-Jon
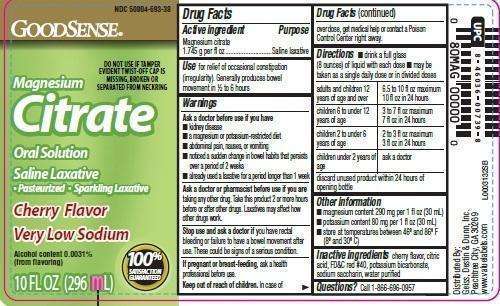
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- use
- Warnings section for this product
- Ask A Doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- Stop use and
- If pregnant or breast-feeding
- Keep out of reach of children.
- Directions
- other information
- Inactive ingredients
- Questions or Comments
- Adverse Reaction
- Pincipal Display Panel
FULL PRESCRIBING INFORMATION
Active Ingredient
Magnesium citrate 1.745g per fl oz
Purpose
Saline laxative
use
for relief of occasional constipation (irregularity). Generally produces bowel movement in 1/2 to 6 hours
Warnings section for this product
Warnings
Ask A Doctor before use if you have
- kidney disease
- a magnesium or potassium restricted diet
- abdominal pain, nausea, or vomiting
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
- already used a laxative for a period longer than 1 week
Ask a doctor or pharmacist before use if you are
taking any other drug. Take this product 2 or more hours before or after other drugs. Laxatives may affect how other drugs work.
Stop use and
ask a doctor if you have rectal bleeding or failure to have a bowel movement after use. These could be signs of a serious condition.
If pregnant or breast-feeding
ask health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away
Directions
- drink a full glass (8 ounces) of liquid with each dose
- may be taken as a single daily dose or in divided doses
adults and children 12 years of age and over - 6.5 to 10 fl oz in 24 hours
children 6 to under 12 years of age - 3 to 7 fl oz maximum 7 fl oz in 24 hours
children 2 to under 6 years of age - 2 to 3 fl oz maximum of 3 fl oz in 24 hours
children under 2 years of age - ask a doctor
discard unused product within 24 hours of opening bottle
other information
- magnesium content 290 mg per 1 fl oz (30 mL)
- potassum content 80 mg per 1 fl oz (30 mL)
- store at temperatures between 46º and 86º F (8º and 30º C)
Inactive ingredients
cherry flavor, citric acid, FD+C red #40, potassium bicarbonate, sodium saccharin, water purified
Questions or Comments
Call 1-866-696-0957
Adverse Reaction
Distributed By: Geiss, Destin & Dunn, Inc
385 Highway 74 South
Peachtree City, GA 30269
www.valuelabels.com
Pincipal Display Panel
NDC 50804-693-38
Good Sense
DO NOT USE IF TAMPE EVIDENT TWIST-OFF CAP IS MISSING, BROKEN OR SEPARATED FROM NECKRING
Magnesium
CITRATE
Oral Solution
Saline Laxative
- Pasteurized
- Sparkling Laxative
Cherry Flavor
Very Low Sodium
Alcohol content 0.0031%
100% SATISFACTION GUARANTEED
10 FL OZ (296 mL)
citromaMagesium Citrate SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||