Citalopram Hydrobromide
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- CITALOPRAM HYDROBROMIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- INDICATIONS & USAGE
- CITALOPRAM HYDROBROMIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- CITALOPRAM HYDROBROMIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Suicidality and Antidepressant DrugsWARNINGS: Clinical Worsening and Suicide RiskPRECAUTIONS: Information for PatientsPRECAUTIONS: Pediatric Use
CITALOPRAM HYDROBROMIDE DESCRIPTION
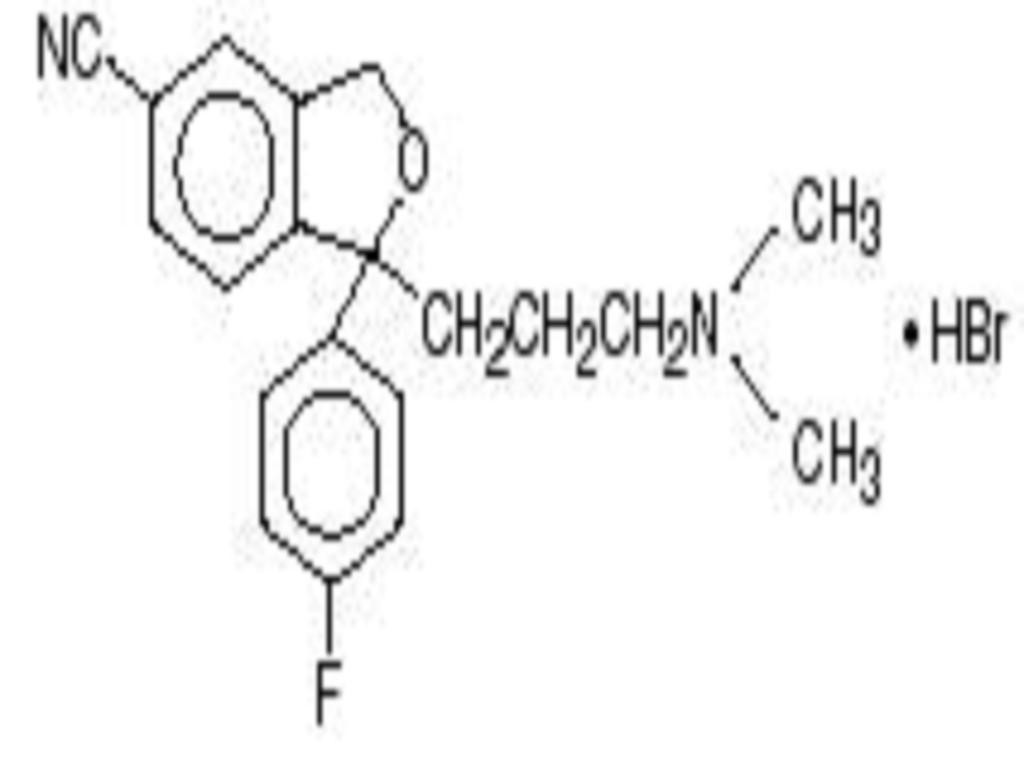
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
PHARMACOKINETICS
Absorption and Distribution
Metabolism and Elimination
Population Subgroups
Age
Gender
Reduced hepatic function
Reduced renal function
Drug-Drug Interactions
Clinical Efficacy Trials
Comparison of Clinical Trial Results
INDICATIONS & USAGE
CITALOPRAM HYDROBROMIDE CONTRAINDICATIONS
WARNINGS
Clinical Worsening and Suicide RiskScreening Patients for Bipolar Disorder
Potential for Interaction With Monoamine Oxidase Inhibitors
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-Like Reactions
PRECAUTIONS
GeneralDiscontinuation of Treatment With Citalopram
Abnormal Bleeding
Hyponatremia
Activation of Mania/Hypomania
Seizures
Interference With Cognitive and Motor Performance
Use in Patients With Concomitant Illness
INFORMATION FOR PATIENTS
Clinical Worsening and Suicide Risk
LABORATORY TESTS
DRUG INTERACTIONS
Serotonergic DrugsTriptans
CNS Drugs
Alcohol
Monoamine Oxidase Inhibitors (MAOIs)
Drugs That Interfere With Hemostasis (NSAIDs, Aspirin, Warfarin, etc.)
Cimetidine
Digoxin
Lithium
Pimozide
Theophylline
Sumatriptan
Warfarin
Carbamazepine
Triazolam
Ketoconazole
CYP3A4 and 2C19 Inhibitors
Metoprolol
Imipramine and Other Tricyclic Antidepressants (TCAs)
Electroconvulsive Therapy (ECT)
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenesis
Impairment of Fertility
PREGNANCY
Nonteratogenic Effects
LABOR & DELIVERY
NURSING MOTHERS
Nursing Mothers
PEDIATRIC USE
GERIATRIC USE
CITALOPRAM HYDROBROMIDE ADVERSE REACTIONS
Adverse Findings Observed in Short-Term, Placebo-Controlled Trials
Adverse Events Associated With Discontinuation of Treatment
Percentage of Patients DiscontinuingDue to Adverse EventBody System/Adverse EventCitalopramPlacebo(N = 1063)(N = 446)GeneralGastrointestinal DisordersCentral and Peripheral Nervous System DisordersPsychiatric Disorders
Adverse Events Occurring at an Incidence of 2% or More Among Citalopram-Treated Patients
*
(Percentage of Patients Reporting Event)Body System/CitalopramPlaceboAdverse Event(N = 1063)(N = 446)Autonomic Nervous System DisordersCentral & Peripheral Nervous System DisordersGastrointestinal DisordersGeneralMusculoskeletal System DisordersPsychiatric DisordersRespiratory System DisordersUrogenital*
Dose Dependency of Adverse Events
The potential relationship between the dose of citalopram administered and the incidence of adverse events was examined in a fixed-dose study in depressed patients receiving placebo or citalopram 10, 20, 40, and 60 mg. Jonckheere's trend test revealed a positive dose response (p < 0.05) for the following adverse events: fatigue, impotence, insomnia, sweating increased, somnolence, and yawning.
Male and Female Sexual Dysfunction With SSRIs
Although changes in sexual desire, sexual performance, and sexual satisfaction often occur as manifestations of a psychiatric disorder, they may also be a consequence of pharmacologic treatment. In particular, some evidence suggests that SSRIs can cause such untoward sexual experiences.
Reliable estimates of the incidence and severity of untoward experiences involving sexual desire, performance, and satisfaction are difficult to obtain, however, in part because patients and physicians may be reluctant to discuss them. Accordingly, estimates of the incidence of untoward sexual experience and performance cited in product labeling, are likely to underestimate their actual incidence.
The table below displays the incidence of sexual side effects reported by at least 2% of patients taking citalopram in a pool of placebo-controlled clinical trials in patients with depression.
TreatmentCitalopramPlacebo(425 males)(194 males)Abnormal Ejaculation6.1%1%(mostly ejaculatory delay)(males only)(males only)Libido Decreased3.8%< 1%(males only)(males only)Impotence2.8%< 1%(males only)(males only)In female depressed patients receiving citalopram, the reported incidence of decreased libido and anorgasmia was 1.3% (n = 638 females) and 1.1% (n = 252 females), respectively.
There are no adequately designed studies examining sexual dysfunction with citalopram treatment.
Priapism has been reported with all SSRIs.
While it is difficult to know the precise risk of sexual dysfunction associated with the use of SSRIs, physicians should routinely inquire about such possible side effects.
Vital Sign Changes
Citalopram and placebo groups were compared with respect to (1) mean change from baseline in vital signs (pulse, systolic blood pressure, and diastolic blood pressure) and (2) the incidence of patients meeting criteria for potentially clinically significant changes from baseline in these variables. These analyses did not reveal any clinically important changes in vital signs associated with citalopram treatment. In addition, a comparison of supine and standing vital sign measures for citalopram and placebo treatments indicated that citalopram treatment is not associated with orthostatic changes.
Weight Changes
Patients treated with citalopram in controlled trials experienced a weight loss of about 0.5 kg compared to no change for placebo patients.
Laboratory Changes
Citalopram and placebo groups were compared with respect to (1) mean change from baseline in various serum chemistry, hematology, and urinalysis variables, and (2) the incidence of patients meeting criteria for potentially clinically significant changes from baseline in these variables. These analyses revealed no clinically important changes in laboratory test parameters associated with citalopram treatment.
ECG Changes
Electrocardiograms from citalopram (N = 802) and placebo (N = 241) groups were compared with respect to (1) mean change from baseline in various ECG parameters, and (2) the incidence of patients meeting criteria for potentially clinically significant changes from baseline in these variables. The only statistically significant drug-placebo difference observed was a decrease in heart rate for citalopram of 1.7 bpm compared to no change in heart rate for placebo. There were no observed differences in QT or other ECG intervals.
Other Events Observed During the Premarketing Evaluation of Citalopram HBr
Following is a list of WHO terms that reflect treatment-emergent adverse events, as defined in the introduction to the ADVERSE REACTIONS section, reported by patients treated with citalopram at multiple doses in a range of 10 to 80 mg/day during any phase of a trial within the premarketing database of 4422 patients. All reported events are included except those already listed in TABLE 3 or elsewhere in labeling, those events for which a drug cause was remote, those event terms which were so general as to be uninformative, and those occurring in only one patient. It is important to emphasize that, although the events reported occurred during treatment with citalopram, they were not necessarily caused by it.
Cardiovascular - Frequent: tachycardia, postural hypotension, hypotension. Infrequent: hypertension, bradycardia, edema (extremities), angina pectoris, extrasystoles, cardiac failure, flushing, myocardial infarction, cerebrovascular accident, myocardial ischemia. Rare: transient ischemic attack, phlebitis, atrial fibrillation, cardiac arrest, bundle branch block.
Central and Peripheral Nervous System Disorders - Frequent: paresthesia, migraine. Infrequent: hyperkinesia, vertigo, hypertonia, extrapyramidal disorder, leg cramps, involuntary muscle contractions, hypokinesia, neuralgia, dystonia, abnormal gait, hypesthesia, ataxia. Rare: abnormal coordination, hyperesthesia, ptosis, stupor.
Endocrine Disorders - Rare: hypothyroidism, goiter, gynecomastia.
Gastrointestinal Disorders - Frequent: saliva increased, flatulence. Infrequent: gastritis, gastroenteritis, stomatitis, eructation, hemorrhoids, dysphagia, teeth grinding, gingivitis, esophagitis. Rare: colitis, gastric ulcer, cholecystitis, cholelithiasis, duodenal ulcer, gastroesophageal reflux, glossitis, jaundice, diverticulitis, rectal hemorrhage, hiccups.
General - Infrequent: hot flushes, rigors, alcohol intolerance, syncope, influenza-like symptoms. Rare: hay fever.
Hemic and Lymphatic Disorders - Infrequent: purpura, anemia, epistaxis, leukocytosis, leucopenia, lymphadenopathy. Rare: pulmonary embolism, granulocytopenia, lymphocytosis, lymphopenia, hypochromic anemia, coagulation disorder, gingival bleeding.
Metabolic and Nutritional Disorders - Frequent: decreased weight, increased weight. Infrequent: increased hepatic enzymes, thirst, dry eyes, increased alkaline phosphatase, abnormal glucose tolerance. Rare: bilirubinemia, hypokalemia, obesity, hypoglycemia, hepatitis, dehydration.
Musculoskeletal System Disorders - Infrequent: arthritis, muscle weakness, skeletal pain. Rare: bursitis, osteoporosis.
Psychiatric Disorders - Frequent: impaired concentration, amnesia, apathy, depression, increased appetite, aggravated depression, suicide attempt, confusion. Infrequent: increased libido, aggressive reaction, paroniria, drug dependence, depersonalization, hallucination, euphoria, psychotic depression, delusion, paranoid reaction, emotional lability, panic reaction, psychosis. Rare: catatonic reaction, melancholia.
Reproductive Disorders/Female* - Frequent: amenorrhea. Infrequent: galactorrhea, breast pain, breast enlargement, vaginal hemorrhage.
* % based on female subjects only: 2955
Respiratory System Disorders - Frequent: coughing. Infrequent: bronchitis, dyspnea, pneumonia. Rare: asthma, laryngitis, bronchospasm, pneumonitis, sputum increased.
Skin and Appendages Disorders - Frequent: rash, pruritus. Infrequent: photosensitivity reaction, urticaria, acne, skin discoloration, eczema, alopecia, dermatitis, skin dry, psoriasis. Rare: hypertrichosis, decreased sweating, melanosis, keratitis, cellulitis, pruritus ani.
Special Senses - Frequent: accommodation abnormal, taste perversion. Infrequent: tinnitus, conjunctivitis, eye pain. Rare: mydriasis, photophobia, diplopia, abnormal lacrimation, cataract, taste loss.
Urinary System Disorders - Frequent: polyuria. Infrequent: micturition frequency, urinary incontinence, urinary retention, dysuria. Rare: facial edema, hematuria, oliguria, pyelonephritis, renal calculus, renal pain.
Other Events Observed During the Postmarketing Evaluation of Citalopram HBr
It is estimated that over 30 million patients have been treated with citalopram since market introduction. Although no causal relationship to citalopram treatment has been found, the following adverse events have been reported to be temporally associated with citalopram treatment, and have not been described elsewhere in labeling: acute renal failure, akathisia, allergic reaction, anaphylaxis, angioedema, choreoathetosis, chest pain, delirium, dyskinesia, ecchymosis, epidermal necrolysis, erythema multiforme, gastrointestinal hemorrhage, glaucoma, grand mal convulsions, hemolytic anemia, hepatic necrosis, myoclonus, nystagmus, pancreatitis, priapism, prolactinemia, prothrombin decreased, QT prolonged, rhabdomyolysis, spontaneous abortion, thrombocytopenia, thrombosis, ventricular arrhythmia, torsade de pointes, and withdrawal syndrome.
DRUG ABUSE AND DEPENDENCE
Controlled Substance ClassPhysical and Psychological Dependence
OVERDOSAGE
Human ExperienceManagement of Overdose
DOSAGE & ADMINISTRATION
Initial TreatmentSpecial Populations
Treatment of Pregnant Women During the Third Trimester
Maintenance Treatment
Discontinuation of Treatment With Citalopram Tablets USP
Switching Patients to or From a Monoamine Oxidase Inhibitor
STORAGE AND HANDLING
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
Retinal Changes in RatsCardiovascular Changes in Dogs
SPL MEDGUIDE
MEDICATION GUIDE-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
-
● What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
-
● Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
-
● Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
-
● Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling very agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● What else do I need to know about antidepressant medicines?
-
● Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
-
● Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
-
● Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
-
● Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
-
● Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Citalopram HydrobromideCitalopram Hydrobromide TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!