Citalopram
State of Florida DOH Central Pharmacy
Citalopram Tablets, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- Suicidality and Antidepressant Drugs
- CITALOPRAM DESCRIPTION
- CLINICAL PHARMACOLOGY
- CITALOPRAM INDICATIONS AND USAGE
- CITALOPRAM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- General
- Discontinuation of Treatment with Citalopram Tablets
- Abnormal Bleeding
- Hyponatremia
- Activation of Mania/Hypomania
- Seizures
- Interference with Cognitive and Motor Performance
- Use in Patients with Concomitant Illness
- Information for Patients
- Laboratory Tests
- Drug Interactions
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenesis
- Mutagenesis
- Impairment of Fertility
- Pregnancy
- Nonteratogenic Effects
- Labor and Delivery
- Nursing Mothers
- Pediatric Use
- CITALOPRAM ADVERSE REACTIONS
- Adverse Findings Observed in Short-Term, Placebo-Controlled Trials
- Adverse Events Associated with Discontinuation of Treatment
- Adverse Events Occurring at an Incidence of 2% or More Among Citalopram-Treated Patients
- Dose Dependency of Adverse Events
- Male and Female Sexual Dysfunction with SSRIs
- Vital Sign Changes
- Weight Changes
- Laboratory Changes
- ECG Changes
- Other Events Observed During the Premarketing Evaluation of Citalopram Tablets
- Other Events Observed During the Postmarketing Evaluation of Citalopram Tablets
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- CITALOPRAM DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- ANIMAL TOXICOLOGY
- Medication Guide
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 40 mg Unit-of-Use Pack (30 Tablets)
FULL PRESCRIBING INFORMATION
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of citalopram tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Citalopram is not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use.)
CITALOPRAM DESCRIPTION
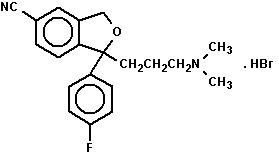
20222
CLINICAL PHARMACOLOGY
Pharmacodynamics
In vitro in vivo
1A, 2A12,121
Pharmacokinetics
Absorption and Distribution
Metabolism and Elimination
In vitro
In vitro
Population Subgroups
DOSAGE AND ADMINISTRATION
-
- DOSAGE AND ADMINISTRATION
-
Drug-Drug Interactions
In vitroin vivoin vivo
Drug Interactions PRECAUTIONS
Clinical Efficacy Trials
Comparison of Clinical Trial Results
CITALOPRAM INDICATIONS AND USAGE
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
CITALOPRAM CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
WARNINGS
Clinical Worsening and Suicide Risk
Table 1
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated |
|---|---|
| |
Increases Compared to Placebo |
| <18 |
14 additional cases |
| 18-24 |
5 additional cases |
| |
Decreases Compared to Placebo |
| 25-64 |
1 fewer case |
| ≥65 |
6 fewer cases |
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
PRECAUTIONS DOSAGE AND ADMINISTRATION—Discontinuation of Treatment with Citalopram Tablets
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers.Such monitoring should include daily observation by families and caregivers.
Screening Patients for Bipolar Disorder
Potential for Interaction with Monoamine Oxidase Inhibitors
In patients receiving serotonin reuptake inhibitor drugs in combination with a monoamine oxidase inhibitor (MAOI), there have been reports of serious, sometimes fatal, reactions including hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation progressing to delirium and coma. These reactions have also been reported in patients who have recently discontinued SSRI treatment and have been started on an MAOI. Some cases presented with features resembling neuroleptic malignant syndrome. Furthermore, limited animal data on the effects of combined use of SSRIs and MAOIs suggest that these drugs may act synergistically to elevate blood pressure and evoke behavioral excitation. Therefore, it is recommended that citalopram tablets should not be used in combination with an MAOI, or within 14 days of discontinuing treatment with an MAOI. Similarly, at least 14 days should be allowed after stopping citalopram tablets before starting an MAOI.
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
PRECAUTIONS
General
Discontinuation of Treatment with Citalopram Tablets
DOSAGE AND ADMINISTRATION
Abnormal Bleeding
Hyponatremia
Geriatric Use
Activation of Mania/Hypomania
Seizures
Interference with Cognitive and Motor Performance
Use in Patients with Concomitant Illness
DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
Information for Patients
Clinical Worsening and Suicide Risk
Laboratory Tests
Drug Interactions
WARNINGS-Serotonin Syndrome PRECAUTIONS - Drug Interactions
WARNINGS - Serotonin Syndrome
-
- CONTRAINDICATIONS WARNINGS
max
-
max
-
-
-
-
-
-max
- In vitro
-
- In vitro
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
22
Mutagenesis
in vitroin vitroin vitro in vitro/in vivoin vitro in vivo
Impairment of Fertility
2
Pregnancy
222
222
Nonteratogenic Effects
WARNINGS
DOSAGE AND ADMINISTRATION
Labor and Delivery
Nursing Mothers
Pediatric Use
BOX WARNING WARNINGS–-Clinical Worsening and Suicide Risk
Geriatric Use
DOSAGE AND ADMINISTRATION
PRECAUTIONS, Hyponatremia
CLINICAL PHARMACOLOGY
DOSAGE AND ADMINISTRATION
CITALOPRAM ADVERSE REACTIONS
Adverse Findings Observed in Short-Term, Placebo-Controlled Trials
Adverse Events Associated with Discontinuation of Treatment
TABLE 2
| Body System/Adverse Event | Percentage of Patients Discontinuing Due to Adverse Event |
|
|---|---|---|
| Citalopram (N=1063) |
Placebo (N=446) |
|
|
General
|
|
|
| Asthenia |
1% |
<1% |
|
Gastrointestinal Disorders
|
|
|
| Nausea |
4% |
0% |
| Dry Mouth |
1% |
<1% |
| Vomiting |
1% |
0% |
|
Central and Peripheral
Nervous System Disorders |
|
|
| Dizziness |
2% |
<1% |
|
Psychiatric Disorders
|
|
|
| Insomnia |
3% |
1% |
| Somnolence |
2% |
1% |
| Agitation |
1% |
<1% |
Adverse Events Occurring at an Incidence of 2% or More Among Citalopram-Treated Patients
Table 3
TABLE 3
| Body System/Adverse Event | (Percentage of Patients Reporting Event) |
|
|---|---|---|
| Citalopram Tablets (N=1063) |
Placebo (N=446) |
|
| * Events reported by at least 2% of patients treated with citalopram tablets are reported, except for the following events which had an incidence on placebo ≥ citalopram tablets: headache, asthenia, dizziness, constipation, palpitation, vision abnormal, sleep disorder, nervousness, pharyngitis, micturition disorder, back pain. 1 Denominator used was for females only (N=638 citalopram tablets; N=252 placebo). 2 Primarily ejaculatory delay. 3 Denominator used was for males only (N=425 citalopram tablets; N=194 placebo). |
||
|
Autonomic Nervous
System Disorders |
|
|
| Dry Mouth |
20% |
14% |
| Sweating Increased |
11% |
9% |
|
Central & Peripheral
Nervous System Disorders |
|
|
| Tremor |
8% |
6% |
|
Gastrointestinal Disorders
|
|
|
| Nausea |
21% |
14% |
| Diarrhea |
8% |
5% |
| Dyspepsia |
5% |
4% |
| Vomiting |
4% |
3% |
| Abdominal Pain |
3% |
2% |
|
General
|
|
|
| Fatigue |
5% |
3% |
| Fever |
2% |
<1% |
|
Musculoskeletal System
Disorders |
|
|
| Arthralgia |
2% |
1% |
| Myalgia |
2% |
1% |
|
Psychiatric Disorder
|
|
|
| Somnolence |
18% |
10% |
| Insomnia |
15% |
14% |
| Anxiety |
4% |
3% |
| Anorexia |
4% |
2% |
| Agitation |
3% |
1% |
| Dysmenorrhea1
|
3% |
2% |
| Libido Decreased |
2% |
<1% |
| Yawning |
2% |
<1% |
|
Respiratory System
Disorders |
|
|
| Upper Respiratory Tract Infection |
5% |
4% |
| Rhinitis |
5% |
3% |
| Sinusitis |
3% |
<1% |
|
Urogenital
|
|
|
| Ejaculation Disorder2,3
|
6% |
1% |
| Impotence3
|
3% |
<1% |
Dose Dependency of Adverse Events
Male and Female Sexual Dysfunction with SSRIs
| Treatment | Citalopram Tablets (425 males) |
Placebo (194 males) |
|---|---|---|
| Abnormal Ejaculation (mostly ejaculatory delay) |
6.1% (males only) |
1% (males only) |
| Libido Decreased |
3.8% (males only) |
<1% (males only) |
| Impotence |
2.8% (males only) |
<1% (males only) |
Vital Sign Changes
Weight Changes
Laboratory Changes
ECG Changes
Other Events Observed During the Premarketing Evaluation of Citalopram Tablets
ADVERSE REACTIONS Table 3
Cardiovascular - Frequent:Infrequent:Rare:
Central and Peripheral Nervous System Disorders - Frequent:Infrequent:Rare:
Endocrine Disorders - Rare:
Gastrointestinal Disorders - Frequent:Infrequent:Rare:
General Infrequent: Rare:
Hemic and Lymphatic Disorders - Infrequent:Rare:
Metabolic and Nutritional Disorders - Frequent:Infrequent:Rare:
Musculoskeletal System Disorders - InfrequentRare
Psychiatric Disorders - Frequent:Infrequent:Rare:
Reproductive Disorders/Female* - Frequent:Infrequent
*
Respiratory System Disorders - Frequent: Infrequent: Rare:
Skin and Appendages Disorders - Frequent:Infrequent:Rare:
Special Senses - Frequent:Infrequent:Rare:
Urinary System Disorders - Frequent:Infrequent:Rare:
Other Events Observed During the Postmarketing Evaluation of Citalopram Tablets
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class
Physical and Psychological Dependence
OVERDOSAGE
Human Experience
Management of Overdose
CITALOPRAM DOSAGE AND ADMINISTRATION
Initial Treatment
Special Populations
Treatment of Pregnant Women During the Third Trimester
PRECAUTIONS
Maintenance Treatment
Clinical Trials CLINICAL PHARMACOLOGY
Discontinuation of Treatment with Citalopram Tablets
PRECAUTIONS
Switching Patients To or From a Monoamine Oxidase Inhibitor
CONTRAINDICATIONS WARNINGS
HOW SUPPLIED
10 mg Tablets –
20 mg Tablets –
40 mg Tablets –
They are supplied by State of Florida DOH Central Pharmacy as follows:
| NDC | Strength | Quantity/Form | Color | Source Prod. Code |
| 53808-0227-1 | 40 mg | 30 Tablets in a Blister Pack | WHITE | 65862-007 |
Store at
ANIMAL TOXICOLOGY
Retinal Changes in Rats
22
Cardiovascular Changes in Dogs
2
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
This Product was Repackaged By:
State of Florida DOH Central Pharmacy
104-2 Hamilton Park Drive
Tallahassee, FL 32304
United States
Medication Guide
Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Talk to your, or your family member’s, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
- How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child’s healthcare provider for more information.
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
This Product was Repackaged By:
State of Florida DOH Central Pharmacy
104-2 Hamilton Park Drive
Tallahassee, FL 32304
United States
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 40 mg Unit-of-Use Pack (30 Tablets)
NDC 53808-0227-1
Citalopram Tablets, USP
40 mg
PHARMACIST: PLEASE DISPENSE WITH
MEDICATION GUIDE PROVIDED SEPARATELY
Rx only 30 Tablets
PLACE PHARMACY LABEL HERE
DO NOT WRAP LABEL AROUND EDGES
AUROBINDO
_613768da.jpg)
CitalopramCitalopram Hydrobromide TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!