ciprofloxacin
PD-Rx Pharmaceuticals, Inc.
PD-Rx Pharmaceuticals, Inc.
Ciprofloxacin Tablets 250 mg, 500 mg and 750 mg
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING:
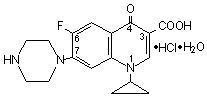
- CIPROFLOXACIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- CIPROFLOXACIN INDICATIONS AND USAGE
- CIPROFLOXACIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CIPROFLOXACIN ADVERSE REACTIONS
- OVERDOSAGE
- HOW SUPPLIED
- ANIMAL PHARMACOLOGY
- CLINICAL STUDIES
- REFERENCES:
- Ciprofloxacin Tablets USP, 250 mg, 500 mg and 750 mg
- What is the most important information I should know about Ciprofloxacin Tablets USP ?
- What is Ciprofloxacin Tablets USP?
- Who should not take Ciprofloxacin Tablets USP?
- What should I tell my healthcare provider before taking Ciprofloxacin Tablets USP?
- How should I take Ciprofloxacin Tablets USP?
- What should I avoid while taking Ciprofloxacin Tablets USP?
- What are the possible side effects of Ciprofloxacin Tablets USP?
- How should I store Ciprofloxacin Tablets USP?
- General Information about Ciprofloxacin Tablets USP
- What are the ingredients in Ciprofloxacin Tablets USP?
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg (100 Tablet Bottle)
FULL PRESCRIBING INFORMATION
WARNING:
Fluoroquinolones, including CIPROFLOXACIN TABLETS USP, 250 mg, 500 mg and 750 mg, are
associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further
increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and
in patients with kidney, heart or lung transplants (See WARNINGS).
CIPROFLOXACIN DESCRIPTION
1718332
171833
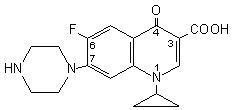
CLINICAL PHARMACOLOGY
Absorption:
|
Dose (mg) |
Maximum Serum Concentrations (μg/mL) |
Area Under Curve (AUC) (μg•hr/mL) |
|---|---|---|
| 250 500 750 1000 |
1.2 2.4 4.3 5.4 |
4.8 11.6 20.2 30.8 |
max
| Steady-state Pharmacokinetic Parameters Following Multiple Oral and I.V. Doses |
||||
|---|---|---|---|---|
| Parameters AUC (μg•hr/mL) Cmax (μg/mL) |
500 mg q12h, P.O. 13.7a 2.97 |
400 mg q12h, I.V. 12.7a 4.56 |
750 mg q12h, P.O. 31.6b 3.59 |
400 mg q8h, I.V. 32.9c 4.07 |
b0-12h
c0-8h
Distribution:
Metabolism:
CONTRAINDICATIONS ; WARNINGS; PRECAUTIONS: Drug Interactions
Excretion:
Drug-drug Interactions:
PRECAUTIONS
CONTRAINDICATIONS.
WARNINGS: PRECAUTIONS
Special Populations:
max PRECAUTIONS: Geriatric Use
DOSAGE AND ADMINISTRATION
MICROBIOLOGY
in vitro In vitro
in vitro.
in vitro INDICATIONS AND USAGE
Aerobic gram-positive microorganisms
Enterococcus faecalis
Staphylococcus aureus
Staphylococcus epidermidis
Staphylococcus saprophyticus
Streptococcus pneumoniae
Streptococcus pyogenes
Aerobic gram-negative microorganisms
Campylobacter jejuni Proteus mirabilis
Citrobacter diversus Proteus vulgaris
Citrobacter freundii Providencia rettgeri
Enterobacter cloacae Providencia stuartii
Escherichia coli Pseudomonas aeruginosa
Haemophilus influenzae Salmonella typhi
Haemophilus parainfluenzae Serratia marcescens
Klebsiella pneumoniae Shigella boydii
Moraxella catarrhalis Shigella dysenteriae
Morganella morganii Shigella flexneri
Neisseria gonorrhoeae Shigella sonnei
Bacillus anthracisin vitro INDICATIONS AND USAGE INHALATIONAL ANTHRAX – ADDITIONAL INFORMATION
in vitro but their clinical significance is unknown
in vitro
Aerobic gram-positive microorganisms
Staphylococcus haemolyticus
Staphylococcus hominis
Streptococcus pneumoniae
Aerobic gram-negative microorganisms
Acinetobacter Iwoffii Pasteurella multocida
Aeromonas hydrophila Salmonella enteritidis
Edwardsiella tarda Vibrio cholerae
Enterobacter aerogenes Vibrio parahaemolyticus
Klebsiella oxytoca Vibrio vulnificus
Legionella pneumophila Yersinia enterocolitica
Burkholderia cepaciaStenotrophomonas maltophiliaBacteroides fragilisClostridium difficile
Susceptibility Tests
Dilution Techniques:
1
Haemophilus influenzaeHaemophilus parainfluenzaeNeisseria gonorrhoeaea
| MIC (μg/mL) | Interpretation |
|---|---|
| ≤ 1 |
Susceptible (S) |
| 2 |
Intermediate (I) |
| ≥ 4 |
Resistant (R) |
a
Haemophilus influenzae Haemophilus parainfluenzaeb
| MIC (μg/mL) | Interpretation |
|---|---|
| ≤ 1 |
Susceptible (S) |
b Haemophilus influenzae Haemophilus parainfluenzae Haemophilus 1
Neisseria gonorrhoeaec
| MIC (μg/mL) | Interpretation |
|---|---|
| ≤ 0.06 |
Susceptible (S) |
| 0.12 – 0.5 |
Intermediate (I) |
| ≥ 1
|
Resistant (R) |
c
| Organism | MIC (μg/mL) | |
|---|---|---|
|
E. faecalis
|
ATCC 29212 |
0.25 – 2 |
|
E. coli
|
ATCC 25922 |
0.004 – 0.015 |
| H. influenzae a | ATCC 49247 |
0.004 – 0.03 |
|
P. aeruginosa
|
ATCC 27853 |
0.25 – 1.0 |
|
S. aureus
|
ATCC 29213 |
0.12 – 0.5 |
|
C. jejuni
b
|
ATCC 33560 |
0.06 – 0.25 and 0.03 – 0.12 |
|
N. gonorrhoeae
c
|
ATCC 49226 |
0.001– 0.008 |
a H. influenzae Haemophilus 1
b C. jejuni 2
c N. gonorrhoeae 23
Diffusion Techniques:
3
EnterobacteriaceaeEnterococcus faecalisStaphylococcusStreptococcus pneumoniaeStreptococcus pyogenesPseudomonas aeruginosaa
| Zone Diameter (mm) | Interpretation |
|---|---|
| ≥ 21 |
Susceptible (S) |
| 16 – 20 |
Intermediate (I) |
| ≤ 15 |
Resistant (R) |
a 2
Haemophilus influenzae Haemophilus parainfluenzaeb
| Zone Diameter (mm) | Interpretation |
|---|---|
| ≥ 21 |
Susceptible (S) |
b Haemophilus influenzae Haemophilus parainfluenzae Haemophilus 3
Neisseria gonorrhoeaec
| Zone Diameter (mm) | Interpretation |
|---|---|
| ≥41 |
Susceptible (S) |
| 28 – 40 |
Intermediate (I) |
| ≤ 27 |
Resistant (R) |
c
| Organism | Zone Diameter (mm) | |
|---|---|---|
|
E. coli
|
ATCC 25922 |
30-40 |
|
H. influenzae
a
|
ATCC 49247 |
34-42 |
|
N. gonorrhoeae
b
|
ATCC 49226 |
48-58 |
|
P. aeruginosa
|
ATCC 27853 |
25-33 |
|
S. aureus
|
ATCC 25923 |
22-30 |
a H. influenzae Haemophilus 3
b N. gonorrhoeae
CIPROFLOXACIN INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Adult Patients:
Urinary Tract Infections Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Serratia marcescens, Proteus mirabilis, Providencia rettgeri, Morganella morganii, Citrobacter diversus, Citrobacter freundii, Pseudomonas aeruginosa, Staphylococcus epidermidis, Staphylococcus saprophyticus, Enterococcus faecalis.
Acute Uncomplicated Cystitis in females Escherichia coli Staphylococcus saprophyticus.
Chronic Bacterial Prostatitis Escherichia coli Proteus mirabilis.
Lower Respiratory Tract Infections Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis, Pseudomonas aeruginosa, Haemophilus influenzae, Haemophilus parainfluenzae, Streptococcus pneumoniae. Moraxella catarrhalis
Streptococcus pneumoniae.
Acute Sinusitis Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis.
Skin and Skin Structure Infections Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis, Proteus vulgaris, Providencia stuartii, Morganella morganii, Citrobacter freundii, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes.
Bone and Joint Infections Enterobacter cloacae, Serratia marcescens, Pseudomonas aeruginosa.
Complicated Intra-Abdominal Infections Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, Bacteroides fragilis.
Infectious Diarrhea Escherichia coli Campylobacter jejuni, Shigella boydii†, Shigella dysenteriae, Shigella flexneri Shigella sonnei†
Typhoid Fever (Enteric Fever) Salmonella typhi.
Uncomplicated cervical and urethral gonorrhea Neisseria gonorrhoeae.
Pediatric patients (1 to 17 years of age):
Complicated Urinary Tract Infections and Pyelonephritis Escherichia coli.
WARNINGS, PRECAUTIONS, Pediatric Use, ADVERSE REACTIONS CLINICAL STUDIES
ANIMAL PHARMACOLOGY
Adult and Pediatric Patients:
Inhalational anthrax Bacillus anthracis.
5 INHALATIONAL ANTHRAX – ADDITIONAL INFORMATION
†
Pseudomonas aeruginosa
CIPROFLOXACIN CONTRAINDICATIONS
PRECAUTIONS: Drug Interactions .
WARNINGS
Tendinopathy and Tendon Rupture:
Fluoroquinolones, including Ciprofloxacin Tablets USP, 250 mg, 500 mg and 750 mg, are associated with an increased risk of tendinitis and tendon rupture in all ages. This adverse reaction most frequently involves the Achilles tendon, and rupture of the Achilles tendon may require surgical repair. Tendinitis and tendon rupture in the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendon sites have also been reported. The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Factors, in addition to age and corticosteroid use, that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have also occurred in patients taking fluoroquinolones who do not have the above risk factors. Tendon rupture can occur during or after completion of therapy; cases occurring up to several months after completion of therapy have been reported. Ciprofloxacin Tablets USP, 250 mg, 500 mg and 750 mg should be discontinued if the patient experiences pain, swelling, inflammation or rupture of a tendon. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug.
Pregnant Women:
THE SAFETY AND EFFECTIVENESS OF CIPROFLOXACIN IN PREGNANT AND LACTATING WOMEN HAVE NOT BEEN ESTABLISHED. PRECAUTIONS: Pregnancy, Nursing Mothers
Pediatrics:
INDICATIONS AND USAGE ADVERSE REACTIONS
ANIMAL PHARMACOLOGY
Cytochrome P450 (CYP450):
Central Nervous System Disorders:
PRECAUTIONS: General, Information for Patients, Drug Interactions ADVERSE REACTIONS
Theophylline:
SERIOUS AND FATAL REACTIONS HAVE BEEN REPORTED IN PATIENTS RECEIVING CONCURRENT ADMINISTRATION OF CIPROFLOXACIN AND THEOPHYLLINE.
Hypersensitivity Reactions:
- fever, rash, or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome);
- vasculitis; arthralgia; myalgia; serum sickness;
- allergic pneumonitis;
- interstitial nephritis; acute renal insufficiency or failure;
- hepatitis; jaundice; acute hepatic necrosis or failure;
- anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
The drug should be discontinued immediately at the first appearance of a skin rash, jaundice, or any other sign of hypersensitivity and supportive measures instituted (See PRECAUTIONS: Information for Patients and ADVERSE REACTIONS ).
Pseudomembranous Colitis:
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Ciprofloxacin Tablets USP, 250 mg, 500 mg and 750 mg, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Peripheral neuropathy:
Syphilis:
PRECAUTIONS
General:
ANIMAL PHARMACOLOGY
Central Nervous System:
WARNINGS, Information for Patients, Drug Interactions
Renal Impairment:
DOSAGE AND ADMINISTRATION
Photosensitivity/Phototoxicity:
ADVERSE REACTIONS/Post-Marketing Adverse Events
Information for Patients:
- to contact their healthcare provider if they experience pain, swelling, or inflammation of a tendon, or weakness or inability to use one of their joints; rest and refrain from exercise; and discontinue Ciprofloxacin Tablets USP, 250 mg, 500 mg and 750 mg treatment. The risk of severe tendon disorder with fluoroquinolones is higher in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
- that antibacterial drugs including Ciprofloxacin Tablets USP, 250 mg, 500 mg and 750 mg should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Ciprofloxacin Tablets USP, 250 mg, 500 mg and 750 mg is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Ciprofloxacin Tablets USP, 250 mg, 500 mg and 750 mg or other antibacterial drugs in the future.
- that ciprofloxacin may be taken with or without meals and to drink fluids liberally. As with other quinolones, concurrent administration of ciprofloxacin with magnesium/aluminum antacids, or sucralfate, Videx® (didanosine) chewable/buffered tablets or pediatric powder, other highly buffered drugs, or with other products containing calcium, iron or zinc should be avoided. Ciprofloxacin may be taken two hours before or six hours after taking these products. Ciprofloxacin should not be taken with dairy products (like milk or yogurt) or calcium-fortified juices alone since absorption of ciprofloxacin may be significantly reduced; however, ciprofloxacin may be taken with a meal that contains these products.
- that ciprofloxacin may be associated with hypersensitivity reactions, even following a single dose, and to discontinue the drug at the first sign of a skin rash or other allergic reaction.
- that photosensitivity/phototoxicity has been reported in patients receiving quinolones. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking quinolones. If patients need to be outdoors while using quinolones, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, patients should contact their physician.
- that peripheral neuropathies have been associated with ciprofloxacin use. If symptoms of peripheral neuropathy including pain, burning, tingling, numbness and/or weakness develop, they should discontinue treatment and contact their physicians.
- that ciprofloxacin may cause dizziness and lightheadedness; therefore, patients should know how they react to this drug before they operate an automobile or machinery or engage in activities requiring mental alertness or coordination.
- that ciprofloxacin increases the effects of tizanidine (Zanaflex®). Patients should not use ciprofloxacin if they are already taking tizanidine.
- that ciprofloxacin may increase the effects of theophylline and caffeine. There is a possibility of caffeine accumulation when products containing caffeine are consumed while taking quinolones.
- that convulsions have been reported in patients receiving quinolones, including ciprofloxacin, and to notify their physician before taking this drug if there is a history of this condition.
- that ciprofloxacin has been associated with an increased rate of adverse events involving joints and surrounding tissue structures (like tendons) in pediatric patients (less than 18 years of age). Parents should inform their child’s physician if the child has a history of joint-related problems before taking this drug. Parents of pediatric patients should also notify their child’s physician of any joint-related problems that occur during or following ciprofloxacin therapy. (See WARNINGS, PRECAUTIONS, Pediatric Use and ADVERSE REACTIONS .)
- that diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Drug Interactions:
max
WARNINGS
DOSAGE AND ADMINISTRATION
2
Carcinogenesis, Mutagenesis, Impairment of Fertility:
in vitro
E. coli
79
Saccharomyces cerevisiae
Saccharomyces cerevisiae
in vivo
2
24
2
Pregnancy
Teratogenic Effects
Pregnancy Category C:
8
9 In utero
10in utero
8,9 WARNINGS
22 WARNINGS
Nursing Mothers:
Pediatric Use:
ANIMAL PHARMACOLOGY
Inhalational Anthrax (Post-Exposure)
DOSAGE AND ADMINISTRATION INHALATIONAL ANTHRAX – ADDITIONAL INFORMATION
Complicated Urinary Tract Infection and Pyelonephritis
Ciprofloxacin is indicated for the treatment of complicated urinary tract infections and pyelonephritis due to Escherichia coli. Although effective in clinical trials, ciprofloxacin is not a drug of first choice in the pediatric population due to an increased incidence of adverse events compared to the controls, including events related to joints and/or surrounding tissues. The rates of these events in pediatric patients with complicated urinary tract infection and pyelonephritis within six weeks of follow-up were 9.3% (31/335) versus 6% (21/349) for control agents. The rates of these events occurring at any time up to the one year follow-up were 13.7% (46/335) and 9.5% (33/349), respectively. The rate of all adverse events regardless of drug relationship at six weeks was 41% (138/335) in the ciprofloxacin arm compared to 31% (109/349) in the control arm. (See ADVERSE REACTIONS and CLINICAL STUDIES .)
Cystic Fibrosis
Geriatric Use:
BOXED WARNING , WARNINGS, ADVERSE REACTIONS/Post-Marketing Adverse Event Reports).
CLINICAL PHARMACOLOGY DOSAGE AND ADMINISTRATION
CIPROFLOXACIN ADVERSE REACTIONS
Side Effects in Adult Patients:
Side Effects in Pediatric Patients:
| Ciprofloxacin | Comparator | |
|---|---|---|
| *The study was designed to demonstrate that the arthropathy rate for the ciprofloxacin group did not exceed that of the control group by more than + 6%. At both the 6 week and 1 year evaluations, the 95% confidence interval indicated that it could not be concluded that ciprofloxacin group had findings comparable to the control group. |
||
| All Patients (within 6 weeks) |
31/335 (9.3%) |
21/349 (6%) |
| 95% Confidence Interval* |
(-0.8%, +7.2%) |
|
| Age Group |
|
|
| ≥ 12 months < 24 months |
1/36 (2.8%) |
0/41 |
| ≥ 2 years < 6 years |
5/124 (4%) |
3/118 (2.5%) |
| ≥ 6 years < 12 years |
18/143 (12.6%) |
12/153 (7.8%) |
| ≥ 12 years to 17 years |
7/32 (21.9%) |
6/37 (16.2 %) |
| All Patients (within 1 year) |
46/335 (13.7%) |
33/349 (9.5%) |
| 95% Confidence Interval* |
(-0.6%, + 9.1%) |
|
Post-Marketing Adverse Event Reports:
PRECAUTIONS
INHALATIONAL ANTHRAX – ADDITIONAL INFORMATION .
Adverse Laboratory Changes:
OVERDOSAGE
DOSAGE AND ADMINISTRATION - ADULTS
| ADULT DOSAGE GUIDELINES | ||||
|---|---|---|---|---|
| Infection | Severity | Dose | Frequency | Usual Durations† |
|
* used in conjunction with metronidazole † Generally ciprofloxacin should be continued for at least 2 days after the signs and symptoms of infection have disappeared, except for inhalational anthrax (post-exposure). ** Drug administration should begin as soon as possible after suspected or confirmed exposure. This indication is based on a surrogate endpoint, ciprofloxacin serum concentrations achieved in humans, reasonably likely to predict clinical benefit.4 For a discussion of ciprofloxacin serum concentrations in various human populations, see INHALATIONAL ANTHRAX – ADDITIONAL INFORMATION . |
||||
| Urinary Tract |
Acute Uncomplicated |
250 mg |
q 12 h |
3 Days |
| Mild/Moderate |
250 mg |
q 12 h |
7 to 14 Days |
|
| Severe/Complicated |
500 mg |
q 12 h |
7 to 14 Days |
|
| Chronic Bacterial Prostatits |
Mild/Moderate |
500 mg |
q 12 h |
28 Days |
| Lower Respiratory Tract |
Mild/Moderate |
500 mg |
q 12 h |
7 to 14 days |
| Severe/Complicated |
750 mg |
q 12 h |
7 to 14 days |
|
| Acute Sinusitis |
Mild/Moderate |
500 mg |
q 12 h |
10 days |
| Skin and Skin Structure |
Mild/Moderate |
500 mg |
q 12 h |
7 to 14 Days |
| Severe/Complicated |
750 mg |
q 12 h |
7 to 14 Days |
|
| Bone and Joint |
Mild/Moderate |
500 mg |
q 12 h |
≥4 to 6 weeks |
| Severe/Complicated |
750 mg |
q 12 h |
≥4 to 6 weeks |
|
| Intra-Abdominal*
|
Complicated |
500 mg |
q 12 h |
7 to 14 Days |
| Infectious Diarrhea |
Mild/Moderate/Severe |
500 mg |
q 12 h |
5 to 7 Days |
| Typhoid Fever |
Mild/Moderate |
500 mg |
q 12 h |
10 Days |
| Urethral and Cervical Gonococcal Infections |
Uncomplicated |
250 mg |
single dose |
single dose |
| Inhalational anthrax (post-exposure)**
|
|
500 mg |
q 12 h |
60 Days |
Conversion of I.V. to Oral Dosing in Adults:
CLINICAL PHARMACOLOGY
| Cipro Oral Dosage | Equivalent Cipro I.V. Dosage |
|---|---|
| 250 mg Tablet q 12 h |
200 mg I.V. q 12 h |
| 500 mg Tablet q 12 h |
400 mg I.V. q 12 h |
| 750 mg Tablet q 12 h |
400 mg I.V. q 8 h |
Adults with Impaired Renal Function:
| Creatinine Clearance (mL/min) | Dose |
|---|---|
| > 50 |
See Usual Dosage. |
| 30-50 |
250-500 mg q 12 h |
| 5-29 |
250-500 mg q 18 h |
|
Patients on hemodialysis or Peritoneal dialysis |
250-500 mg q 24 h (after dialysis) |
Weight (kg) x (140 - age)
DOSAGE AND ADMINISTRATION - PEDIATRICS
ADVERSE REACTIONS CLINICAL STUDIES
| Infection | Route of Administration |
Dose (mg/kg) |
Frequency | Total Duration |
|---|---|---|---|---|
| * The total duration of therapy for complicated urinary tract infection and pyelonephritis in the clinical trial was determined by the physician. The mean duration of treatment was 11 days (range 10 to 21 days). ** Drug administration should begin as soon as possible after suspected or confirmed exposure to Bacillus anthracis spores. This indication is based on a surrogate endpoint, ciprofloxacin serum concentrations achieved in humans, reasonably likely to predict clinical benefit.5 For a discussion of ciprofloxacin serum concentrations in various human populations, see INHALATIONAL ANTHRAX – ADDITIONAL INFORMATION . |
||||
| Complicated Urinary Tract or Pyelonephritis |
Intravenous |
6 to 10 mg/kg (maximum 400 mg per dose; not to be exceeded even in patients weighing > 51 kg) |
Every 8 hours |
10-21 days* |
| (patients from 1 to 17 years of age) |
Oral |
10 mg/kg to 20 mg/kg (maximum 750 mg per dose; not to be exceeded even in patients weighing > 51 kg) |
Every 12 hours |
|
| Inhalational Anthrax (Post-Exposure)** |
Intravenous |
10 mg/kg (maximum 400 mg per dose) |
Every 12 hours |
60 days |
| Oral |
15 mg/kg (maximum 500 mg per dose) |
Every 12 hours |
||
2
HOW SUPPLIED
Ciprofloxacin Tablets are available as round biconvex white to slightly yellowish film coated tablets containing 250 mg of ciprofloxacin. The 250 mg tablet is embossed with the word "P" on one side and "250" on reverse side. The 500 mg and 750 mg tablet are available as capsule shaped, white to slightly yellowish film coated tablets with the word "P" embossed on one side and "500" or "750" on reverse side, respectively.
| Strength | NDC Code | Tablet Identification | |
|---|---|---|---|
| Bottles of 50: | 250 mg 500 mg 750 mg |
NDC 16571-411-05 NDC 16571-412-05 NDC 16571-413-05 |
P 250 P 500 P 750 |
| Bottles of 100: | 250 mg 500 mg 750 mg |
NDC 16571-411-10 NDC 16571-412-10 NDC 16571-413-10 |
P 250 P 500 P 750 |
| Bottles of 500: | 250 mg 500 mg 750 mg |
NDC 16571-411-50 NDC 16571-412-50 NDC 16571-413-50 |
P 250 P 500 P 750 |
Store below 30°C (86°F).
Manufactured by:
Unique Pharmaceutical Laboratories
Neelam Centre, Hind Cycle Road
Worli, Mumbai 400 025, India
Distributed by:
PACK Pharmaceuticals, LLC, Buffalo Grove, IL 60089 USA
ANIMAL PHARMACOLOGY
WARNINGS
22
CLINICAL STUDIES
Complicated Urinary Tract Infection and Pyelonephritis – Efficacy in Pediatric Patients:
| Ciprofloxacin | Comparator | |
|---|---|---|
| * Patients with baseline pathogen(s) eradicated and no new infections or superinfections/total number of patients. There were 5.5% (6/211) ciprofloxacin and 9.5% (22/231) comparator patients with superinfections or new infections. |
||
| Randomized Patients |
337 |
352 |
| Per Protocol Patients |
211 |
231 |
| Clinical Response at 5 to 9 Days Post-Treatment |
95.7% (202/211) |
92.6% (214/231) |
| |
95% CI [-1.3%, 7.3%] |
|
| Bacteriologic Eradication by Patient at 5 to 9 Days Post-Treatment* |
84.4% (178/211) |
78.3% (181/231) |
| |
95% CI [-1.3%, 13.1%] |
|
| Bacteriologic Eradication of the Baseline Pathogen at 5 to 9 Days Post-Treatment |
|
|
| Escherichia coli
|
156/178 (88%) |
161/179 (90%) |
INHALATIONAL ANTHRAX IN ADULTS AND PEDIATRICS – ADDITIONAL INFORMATION
DOSAGE AND ADMINISTRATION PRECAUTIONS, Pediatric Use 5
50550B. anthracis max67
B. anthracis
REFERENCES:
- National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically-Fifth Edition. Approved Standard NCCLS Document M7-A5, Vol. 20, No. 2, NCCLS, Wayne, PA, January, 2000.
- Clinical and Laboratory Standards Institute, Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Approved Guideline., CLSI Document M45-A, Vol. 26, No. 19, CLSI, Wayne, PA, 2006.
- National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disk Susceptibility Tests-Seventh Edition. Approved Standard NCCLS Document M2-A7, Vol. 20, No. 1, NCCLS, Wayne, PA, January, 2000.
- Report presented at the FDA’s Anti-Infective Drug and Dermatological Drug Product’s Advisory Committee meeting, March 31, 1993, Silver Spring, MD. Report available from FDA, CDER, Advisors and Consultants Staff, HFD-21, 1901 Chapman Avenue, Room 200, Rockville, MD 20852, USA.
- 21 CFR 314.510 (Subpart H – Accelerated Approval of New Drugs for Life-Threatening Illnesses).
- Kelly DJ, et al. Serum concentrations of penicillin, doxycycline, and ciprofloxacin during prolonged therapy in rhesus monkeys. J Infect Dis 1992; 166:1184-7.
- Friedlander AM, et al. Postexposure prophylaxis against experimental inhalational anthrax. J Infect Dis 1993; 167:1239-42.
- Friedman J, Polifka J. Teratogenic effects of drugs: a resource for clinicians (TERIS). Baltimore, Maryland: Johns Hopkins University Press, 2000:149-195.
- Loebstein R, Addis A, Ho E, et al. Pregnancy outcome following gestational exposure to fluoroquinolones: a multicenter prospective controlled study. Antimicrob Agents Chemother. 1998;42(6):1336-1339.
- Schaefer C, Amoura-Elefant E, Vial T, et al. Pregnancy outcome after prenatal quinolone exposure. Evaluation of a case registry of the European network of teratology information services (ENTIS). Eur J Obstet Gynecol Reprod Biol. 1996;69:83-89.
Ciprofloxacin Tablets USP, 250 mg, 500 mg and 750 mg
Read the Medication Guide that comes with Ciprofloxacin Tablets USP before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about Ciprofloxacin Tablets USP ?
Ciprofloxacin Tablets USP belongs to a class of antibiotics called fluoroquinolones. Ciprofloxacin Tablets USP can cause side effects that may be serious or even cause death. If you get any of the following serious side effects, get medical help right away. Talk with your healthcare provider about whether you should continue to take Ciprofloxacin Tablets USP.
Tendon rupture or swelling of the tendon (tendinitis)
- Tendons are tough cords of tissue that connect muscles to bones.
- Pain, swelling, tears and inflammation of tendons including the back of the ankle (Achilles), shoulder, hand, or other tendon sites can happen in people of all ages who take fluoroquinolone antibiotics, including Ciprofloxacin Tablets USP. The risk of getting tendon problems is higher if you:
- are over 60 years of age
- are taking steroids (corticosteroids)
- have had a kidney, heart or lung transplant
- Swelling of the tendon (tendinitis) and tendon rupture (breakage) have also happened in patients who take fluoroquinolones who do not have the above risk factors.
- Other reasons for tendon ruptures can include:
- physical activity or exercise
- kidney failure
- tendon problems in the past, such as in people with rheumatoid arthritis (RA)
- Call your healthcare provider right away at the first sign of tendon pain, swelling or inflammation. Stop taking Ciprofloxacin Tablets USP until tendinitis or tendon rupture has been ruled out by your healthcare provider. Avoid exercise and using the affected area. The most common area of pain and swelling is the Achilles tendon at the back of your ankle. This can also happen with other tendons. Talk to your healthcare provider about the risk of tendon rupture with continued use of Ciprofloxacin Tablets USP. You may need a different antibiotic that is not a fluoroquinolone to treat your infection.
- Tendon rupture can happen while you are taking or after you have finished taking Ciprofloxacin Tablets USP. Tendon ruptures have happened up to several months after patients have finished taking their fluoroquinolone.
- Get medical help right away if you get any of the following signs or symptoms of a tendon rupture:
- hear or feel a snap or pop in a tendon area
- bruising right after an injury in a tendon area
- unable to move the affected area or bear weight
- See the section “What are the possible side effects of Ciprofloxacin Tablets USP?” for more information about side effects.
What is Ciprofloxacin Tablets USP?
Ciprofloxacin Tablets USP is a fluoroquinolone antibiotic medicine used to treat certain infections caused by certain germs called bacteria.
Children less than 18 years of age have a higher chance of getting bone, joint, or tendon (musculoskeletal) problems such as pain or swelling while taking Ciprofloxacin Tablets USP. Ciprofloxacin Tablets USP should not be used as the first choice of antibiotic medicine in children under 18 years of age.
Ciprofloxacin Tablets USP should not be used in children under 18 years old, except to treat specific serious infections, such as complicated urinary tract infections and to prevent anthrax disease after breathing the anthrax bacteria germ (inhalational exposure). It is not known if Ciprofloxacin Extended Release Tablets are safe and work in children under 18 years of age.
Sometimes infections are caused by viruses rather than by bacteria. Examples include viral infections in the sinuses and lungs, such as the common cold or flu. Antibiotics, including Ciprofloxacin Tablets USP, do not kill viruses.
Call your healthcare provider if you think your condition is not getting better while you are taking Ciprofloxacin Tablets USP.
Who should not take Ciprofloxacin Tablets USP?
Do not take Ciprofloxacin Tablets USP if you:
- have ever had a severe allergic reaction to an antibiotic known as a fluoroquinolone, or are allergic to any of the ingredients in Ciprofloxacin Tablets USP. Ask your healthcare provider if you are not sure. See the list of ingredients in Ciprofloxacin Tablets USP at the end of this Medication Guide.
- also take a medicine called tizanidine (Zanaflex®). Serious side effects from tizanidine are likely to happen.
What should I tell my healthcare provider before taking Ciprofloxacin Tablets USP?
See “What is the most important information I should know about Ciprofloxacin Tablets USP?”
Tell your healthcare provider about all your medical conditions, including if you:
- have tendon problems
- have central nervous system problems (such as epilepsy)
- have nerve problems
- have or anyone in your family has an irregular heartbeat, especially a condition called “QT prolongation”
- have a history of seizures
- have kidney problems. You may need a lower dose of Ciprofloxacin Tablets USP if your kidneys do not work well.
- have rheumatoid arthritis (RA) or other history of joint problems
- have trouble swallowing pills
- are pregnant or planning to become pregnant. It is not known if Ciprofloxacin Tablets USP will harm your unborn child.
- are breast-feeding or planning to breast-feed. Ciprofloxacin Tablets USP passes into breast milk. You and your healthcare provider should decide whether you will take Ciprofloxacin Tablets USP or breast-feed.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal and dietary supplements. Ciprofloxacin Tablets USP and other medicines can affect each other causing side effects. Especially tell your healthcare provider if you take:
- an NSAID (Non-Steroidal Anti-Inflammatory Drug). Many common medicines for pain relief are NSAIDs. Taking an NSAID while you take Ciprofloxacin Tablets USP or other fluoroquinolones may increase your risk of central nervous system effects and seizures. See “What are the possible side effects of Ciprofloxacin Tablets USP?”
- a blood thinner (warfarin, Coumadin®, Jantoven®)
- tizanidine (Zanaflex®). You should not take Ciprofloxacin Tablets USP if you are already taking tizanidine. See “Who should not take Ciprofloxacin Tablets USP?”
- theophylline (Theo-24®, Elixophyllin®, Theochron®, Uniphyl®, Theolair®)
- glyburide (Micronase®, Glynase®, Diabeta®, Glucovance®). See “What are the possible side effects of Ciprofloxacin Tablets USP?”
- phenytoin (Fosphenytoin Sodium®, Cerebyx®, Dilantin-125®, Dilantin®, Extended Phenytoin Sodium®, Prompt Penytoin Sodium®, Phenytek®)
- products that contain caffeine
- a medicine to control your heart rate or rhythm (antiarrhythmics) See “What are the possible side effects of Ciprofloxacin Tablets USP?”
- an anti-psychotic medicine
- a tricyclic antidepressant
- a water pill (diuretic)
- a steroid medicine. Corticosteroids taken by mouth or by injection may increase the chance of tendon injury. See “What is the most important information I should know about Ciprofloxacin Tablets USP?”
- methotrexate (Trexall®)
- Probenecid (Probalan®, Col-probenecid®)
- Metoclopromide (Reglan®, Reglan ODT®)
- Certain medicines may keep Ciprofloxacin Tablets USP from working correctly.
Take Ciprofloxacin Tablets USP either 2 hours before or 6 hours after taking
these products:
- an antacid, multivitamin, or other product that has magnesium, calcium, aluminum, iron, or zinc
- sucralfate (Carafate®)
- didanosine (Videx®, Videx EC®)
Ask your healthcare provider if you are not sure if any of your medicines are listed above.
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take Ciprofloxacin Tablets USP?
- Take Ciprofloxacin Tablets USP exactly as prescribed by your healthcare provider.
- Take Ciprofloxacin Tablets USP in the morning and evening at about the same time each day. Swallow the tablet whole. Do not split, crush or chew the tablet. Tell your healthcare provider if you can not swallow the tablet whole.
- Ciprofloxacin Tablets USP can be taken with or without food.
- Ciprofloxacin Tablets USP should not be taken with dairy products (like milk or yogurt) or calcium-fortified juices alone, but may be taken with a meal that contains these products.
- Drink plenty of fluids while taking Ciprofloxacin Tablets USP.
- Do not skip any doses, or stop taking Ciprofloxacin Tablets USP even if you begin to feel better, until you finish your prescribed treatment, unless:
- you have tendon effects (see “What is the most important information I should know about Ciprofloxacin Tablets USP?” ),
- you have a serious allergic reaction (see “What are the possible side effects of Ciprofloxacin Tablets USP?” ), or
- your healthcare provider tells you to stop.
- This will help make sure that all of the bacteria are killed and lower the chance that the bacteria will become resistant to Ciprofloxacin Tablets USP. If this happens, Ciprofloxacin Tablets USP and other antibiotic medicines may not work in the future.
- If you miss a dose of Ciprofloxacin Tablets USP, take it as soon as you remember. Do not take two doses at the same time, and do not take more than two doses in one day.
- If you take too much, call your healthcare provider or get medical help immediately.
If you have been prescribed Ciprofloxacin Tablets USP after being exposed to anthrax:
- Ciprofloxacin Tablets USP has been approved to lessen the chance of getting anthrax disease or worsening of the disease after you are exposed to the anthrax bacteria germ.
- Take Ciprofloxacin Tablets USP exactly as prescribed by your healthcare provider. Do not stop taking Ciprofloxacin Tablets USP without talking with your healthcare provider. If you stop taking Ciprofloxacin Tablets USP too soon, it may not keep you from getting the anthrax disease.
- Side effects may happen while you are taking Ciprofloxacin Tablets USP. When taking your Ciprofloxacin Tablets USP to prevent anthrax infection, you and your healthcare provider should talk about whether the risks of stopping Ciprofloxacin Tablets USP too soon are more important than the risks of side effects with Ciprofloxacin Tablets USP.
- If you are pregnant, or plan to become pregnant while taking Ciprofloxacin Tablets USP, you and your healthcare provider should decide whether the benefits of taking Ciprofloxacin Tablets USP for anthrax are more important than the risks.
What should I avoid while taking Ciprofloxacin Tablets USP?
- Ciprofloxacin Tablets USP can make you feel dizzy and lightheaded. Do not drive, operate machinery, or do other activities that require mental alertness or coordination until you know how Ciprofloxacin Tablets USP affects you.
- Avoid sunlamps, tanning beds, and try to limit your time in the sun. Ciprofloxacin Tablets USP can make your skin sensitive to the sun (photosensitivity) and the light from sunlamps and tanning beds. You could get severe sunburn, blisters or swelling of your skin. If you get any of these symptoms while taking Ciprofloxacin Tablets USP, call your healthcare provider right away. You should use a sunscreen and wear a hat and clothes that cover your skin if you have to be in sunlight.
What are the possible side effects of Ciprofloxacin Tablets USP?
- Ciprofloxacin Tablets USP can cause side effects that may be serious or even cause death. See “What is the most important information I should know about Ciprofloxacin Tablets USP?”
Other serious side effects of Ciprofloxacin Tablets USP include:
-
Central Nervous System effects: Seizures have been reported in people who take
fluoroquinolone antibiotics including Ciprofloxacin Tablets USP. Tell your
healthcare provider if you have a history of seizures. Ask your healthcare provider
whether taking Ciprofloxacin Tablets USP will change your risk of having a
seizure.
Central Nervous System (CNS) side effects may happen as soon as after taking the first dose of Ciprofloxacin Tablets USP. Talk to your healthcare provider right away if you get any of these side effects, or other changes in mood or behavior:- feel dizzy
- seizures
- hear voices, see things, or sense things that are not there (hallucinations)
- feel restless
- tremors
- feel anxious or nervous
- confusion
- depression
- trouble sleeping
- nightmares
- feel more suspicious (paranoia)
- suicidal thoughts or acts
-
Serious allergic reactions: Allergic reactions can happen in people taking
fluoroquinolones, including Ciprofloxacin Tablets USP, even after only one dose.
Stop taking Ciprofloxacin Tablets USP and get emergency medical help right away if
you get any of the following symptoms of a severe allergic reaction:
- hives
- trouble breathing or swallowing
- swelling of the lips, tongue, face
- throat tightness, hoarseness
- rapid heartbeat
- faint
- yellowing of the skin or eyes. Stop taking Ciprofloxacin Tablets USP and tell your healthcare provider right away if you get yellowing of your skin or white part of your eyes, or if you have dark urine. These can be signs of a serious reaction to Ciprofloxacin Tablets USP (a liver problem).
-
Skin rash
Skin rash may happen in people taking Ciprofloxacin Tablets USP even after only one dose. Stop taking Ciprofloxacin Tablets USP at the first sign of a skin rash and call your healthcare provider. Skin rash may be a sign of a more serious reaction to Ciprofloxacin Tablets USP. -
Serious heart rhythm changes (QT prolongation and torsade de pointes)
Tell your healthcare provider right away if you have a change in your heart beat (a fast or irregular heartbeat), or if you faint. Ciprofloxacin Tablets USP may cause a rare heart problem known as prolongation of the QT interval. This condition can cause an abnormal heartbeat and can be very dangerous. The chances of this event are higher in people:- who are elderly
- with a family history of prolonged QT interval
- with low blood potassium (hypokalemia)
- who take certain medicines to control heart rhythm (antiarrhythmics)
-
Intestine infection (Pseudomembranous colitis)
Pseudomembranous colitis can happen with most antibiotics, including Ciprofloxacin Tablets USP. Call your healthcare provider right away if you get watery diarrhea, diarrhea that does not go away, or bloody stools. You may have stomach cramps and a fever. Pseudomembranous colitis can happen 2 or more months after you have finished your antibiotic. -
Changes in sensation and possible nerve damage (Peripheral Neuropathy)
Damage to the nerves in arms, hands, legs, or feet can happen in people who take fluoroquinolones, including Ciprofloxacin Tablets USP. Talk with your healthcare provider right away if you get any of the following symptoms of peripheral neuropathy in your arms, hands, legs, or feet:- pain
- burning
- tingling
- numbness
- weakness
Ciprofloxacin tablets may need to be stopped to prevent permanent nerve damage. -
Low blood sugar (hypoglycemia)
People who take Ciprofloxacin Tablets USP and other fluoroquinolone medicines with the oral anti-diabetes medicine glyburide (Micronase, Glynase, Diabeta, Glucovance) can get low blood sugar (hypoglycemia) which can sometimes be severe. Tell your healthcare provider if you get low blood sugar with CIPRO. Your antibiotic medicine may need to be changed. -
Sensitivity to sunlight (photosensitivity)
See “What should I avoid while taking Ciprofloxacin Tablets USP?” -
Joint Problems
Increased chance of problems with joints and tissues around joints in children under 18 years old. Tell your child’s healthcare provider if your child has any joint problems during or after treatment with Ciprofloxacin Tablets USP.
The most common side effects of Ciprofloxacin Tablets USP include:- nausea
- headache
- diarrhea
- vomiting
- vaginal yeast infection
- changes in liver function tests
- pain or discomfort in the abdomen
These are not all the possible side effects of Ciprofloxacin Tablets USP. Tell your healthcare provider about any side effect that bothers you, or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Ciprofloxacin Tablets USP?
- Store Ciprofloxacin Tablets USP below 86°F (30°C)
Keep Ciprofloxacin Tablets USP and all medicines out of the reach of children.
General Information about Ciprofloxacin Tablets USP
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Ciprofloxacin Tablets USP for a condition for which it is not prescribed. Do not give Ciprofloxacin Tablets USP to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Ciprofloxacin Tablets USP. If you would like more information about CIPRO, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Ciprofloxacin Tablets USP that is written for healthcare professionals.
For more information 1-(866)-562-4597
What are the ingredients in Ciprofloxacin Tablets USP?
- Active ingredient: ciprofloxacin
- Inactive ingredients: pregelatinized starch, microcrystalline cellulose, colloidal silicon dioxide, crospovidone, magnesium stearate, hypromellose, titanium dioxide, polyethylene glycol and purified water
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Unique Pharmaceutical Laboratories
(A Div. of J. B. Chemicals & Pharmaceuticals Ltd)
Mumbai 400 030, India
Distributed by:
PACK Pharmaceuticals, LLC,
Buffalo Grove, IL 60089 USA
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg (100 Tablet Bottle)
Ciprofloxacin Tablets USP 250 mg*
Attention: Dispense with a Medication Guide
* Each tablet contains:
Ciprofloxacin Hydrochloride USP equivalent to 250 mg of ciprofloxacin.
Dosage:See accompanying package insert for complete information on dosage and administration
Recommedned Storage:Store below 86°F (30°C)

ciprofloxacinciprofloxacin TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||