Ciprofloxacin Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- CIPROFLOXACIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- MICROBIOLOGY
- INDICATIONS & USAGE
- CIPROFLOXACIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- CIPROFLOXACIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- CLINICAL STUDIES
- REFERENCES
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNINGFluoroquinolones, including ciprofloxacin, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants (see WARNINGS).
Fluoroquinolones, including ciprofloxacin, may exacerbate muscle weakness in persons with myasthenia gravis. Avoid ciprofloxacin in patients with known history of myasthenia gravis (see WARNINGS).
CIPROFLOXACIN HYDROCHLORIDE DESCRIPTION
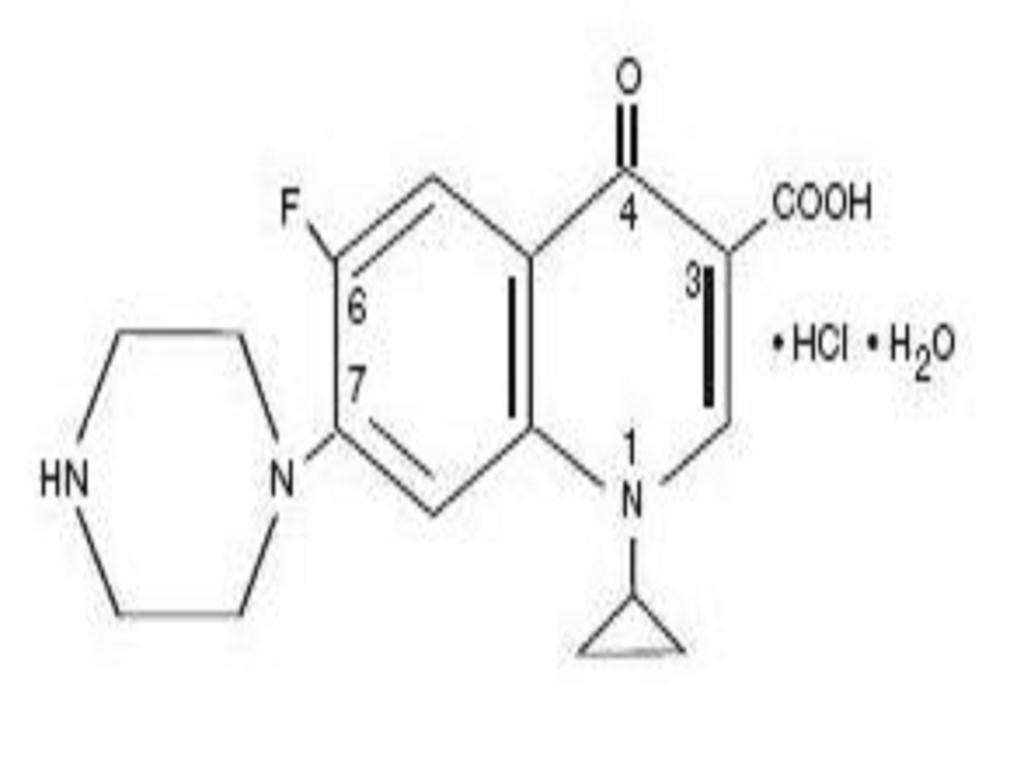
CLINICAL PHARMACOLOGY
AbsorptionDistribution
Metabolism
Excretion
Drug-Drug Interactions
Special Populations
MICROBIOLOGY
Susceptibility Tests
Dilution Techniques
Diffusion Techniques
INDICATIONS & USAGE
Adult Patients
Pediatric patients (1 to 17 years of age)
Adult and Pediatric Patients
CIPROFLOXACIN HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Tendinopathy and Tendon RuptureExacerbation of Myasthenia Gravis
Pregnant Women
Pediatrics
Cytochrome P450 (CYP450)
Central Nervous System Disorders
Theophylline
Hypersensitivity Reactions
Pseudomembranous Colitis
Peripheral Neuropathy
Syphilis
PRECAUTIONS
GeneralCentral Nervous System
Renal Impairment
Photosensitivity/Phototoxicity
INFORMATION FOR PATIENTS
-
●
-
● that fluoroquinolones like ciprofloxacin may cause worsening of myasthenia gravis symptoms, including muscle weakness and breathing problems. Patients should call their healthcare provider right away if they have any worsening muscle weakness or breathing problems.
-
● that antibacterial drugs including ciprofloxacin tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When ciprofloxacin tablets are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ciprofloxacin tablets or other antibacterial drugs in the future.
-
● that ciprofloxacin may be taken with or without meals and to drink fluids liberally. As with other quinolones, concurrent administration of ciprofloxacin with magnesium/aluminum antacids, or sucralfate, Videx
-
● that ciprofloxacin may be associated with hypersensitivity reactions, even following a single dose, and to discontinue the drug at the first sign of a skin rash or other allergic reaction.
-
● that photosensitivity/phototoxicity has been reported in patients receiving quinolones. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking quinolones. If patients need to be outdoors while using quinolones, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, patients should contact their physician.
-
● that peripheral neuropathies have been associated with ciprofloxacin use. If symptoms of peripheral neuropathy including pain, burning, tingling, numbness and/or weakness develop, they should discontinue treatment and contact their physicians.
-
● that ciprofloxacin may cause dizziness and lightheadedness; therefore, patients should know how they react to this drug before they operate an automobile or machinery or engage in activities requiring mental alertness or coordination.
-
● that ciprofloxacin increases the effects of tizanidine (ZanaflexPatients should not use ciprofloxacin if they are already taking tizanidine.
-
● that ciprofloxacin may increase the effects of theophylline and caffeine. There is a possibility of caffeine accumulation when products containing caffeine are consumed while taking quinolones.
-
● that convulsions have been reported in patients receiving quinolones, including ciprofloxacin, and to notify their physician before taking this drug if there is a history of this condition.
-
● that ciprofloxacin has been associated with an increased rate of adverse events involving joints and surrounding tissue structures (like tendons) in pediatric patients (less than 18 years of age). Parents should inform their child's physician if the child has a history of joint-related problems before taking this drug. Parents of pediatric patients should also notify their child's physician of any joint-related problems that occur during or following ciprofloxacin therapy (see WARNINGS, PRECAUTIONS, Pediatric Use and ADVERSEREACTIONS).
-
● that diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
-
●
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category C
NURSING MOTHERS
PEDIATRIC USE
Inhalational Anthrax (Post-Exposure)
Complicated Urinary Tract Infection and Pyelonephritis
Cystic Fibrosis
GERIATRIC USE
CIPROFLOXACIN HYDROCHLORIDE ADVERSE REACTIONS
Adverse Reactions in Adult PatientsAdverse Reactions in Pediatric Patients
Postmarketing Adverse Events
Adverse Laboratory Changes
OVERDOSAGE
DOSAGE & ADMINISTRATION
Conversion of I.V. to Oral Dosing in Adults
Adults with Impaired Renal Function
DOSAGE AND ADMINISTRATION - PEDIATRICS
HOW SUPPLIED
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
CLINICAL STUDIES
Complicated Urinary Tract Infection and PyelonephritisEfficacy in Pediatric PatientsINHALATIONAL ANTHRAX IN ADULTS AND PEDIATRICSADDITIONAL INFORMATION
REFERENCES
SPL MEDGUIDE
CIPROFLOXACIN TABLETS USPPACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Ciprofloxacin HydrochlorideCiprofloxacin Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!