CHLOROTHIAZIDE SODIUM
Chlorothiazide Sodium for Injection, USPFOR THE PREPARATION OF INTRAVENOUS SOLUTIONS
FULL PRESCRIBING INFORMATION: CONTENTS*
- CHLOROTHIAZIDE SODIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- CHLOROTHIAZIDE SODIUM INDICATIONS AND USAGE
- CHLOROTHIAZIDE SODIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CHLOROTHIAZIDE SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- CHLOROTHIAZIDE SODIUM DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON
FULL PRESCRIBING INFORMATION
CHLOROTHIAZIDE SODIUM DESCRIPTION
2 H75342

2 H76342
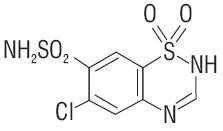
CLINICAL PHARMACOLOGY
Pharmacokinetics and Metabolism
CHLOROTHIAZIDE SODIUM INDICATIONS AND USAGE
Use in Pregnancy
PRECAUTIONS, Pregnancy
CHLOROTHIAZIDE SODIUM CONTRAINDICATIONS
WARNINGS
PRECAUTIONS, Drug Interactions
PRECAUTIONS
General
Laboratory Tests
Drug Interactions
Alcohol, barbiturates, or narcotics
Antidiabetic drugs - (oral agents and insulin)
Other antihypertensive drugs
Corticosteroids, ACTH
Pressor amines (e.g., norepinephrine)
Skeletal muscle relaxants, nondepolarizing (e.g., tubocurarine)
Lithium
Non-steroidal Anti-inflammatory Drugs
Drug/Laboratory Test Interactions
PRECAUTIONS, General
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitroSalmonella typhimuriumAspergillus nidulans
1
1
Pregnancy
Teratogenic Effects
INDICATIONS AND USAGE
Nonteratogenic Effects:
Nursing Mothers
Pediatric Use
Geriatric Use
WARNINGS
CHLOROTHIAZIDE SODIUM ADVERSE REACTIONS
Body as a Whole:
Cardiovascular:
Digestive:
Hematologic:
Hypersensitivity:
Metabolic:PRECAUTIONS
Musculoskeletal:
Nervous System/Psychiatric:
Skin:
Special Senses:
Renal:WARNINGS
Urogenital:
OVERDOSAGE
50
CHLOROTHIAZIDE SODIUM DOSAGE AND ADMINISTRATION
Extravasation must be rigidly avoided. Do not give subcutaneously or intramuscularly.
Directions for Reconstitution
Use aseptic technique. Because chlorothiazide sodium for injection contains no preservative, a fresh solution should be prepared immediately prior to each administration, and the unused portion should be discarded.
HOW SUPPLIED
Storage
DOSAGE AND ADMINISTRATION, Directions for Reconstitution
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Ind. Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON
NDC 47335-330-40
Chlorothiazide Sodium for Injection, USP
500 mg/vial
STERILE
LYOPHILIZED
FOR THE PREPARATION OF INTRAVENOUS SOLUTIONS
Rx only
One Single Dose Vial
SUN PHARMACEUTICAL INDUSTRIES LTD.
CHLOROTHIAZIDE SODIUMCHLOROTHIAZIDE SODIUM INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!