Chlordiazepoxide Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- CHLORDIAZEPOXIDE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- CHLORDIAZEPOXIDE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PEDIATRIC USE
- INFORMATION FOR PATIENTS
- CHLORDIAZEPOXIDE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
CHLORDIAZEPOXIDE HYDROCHLORIDE DESCRIPTION
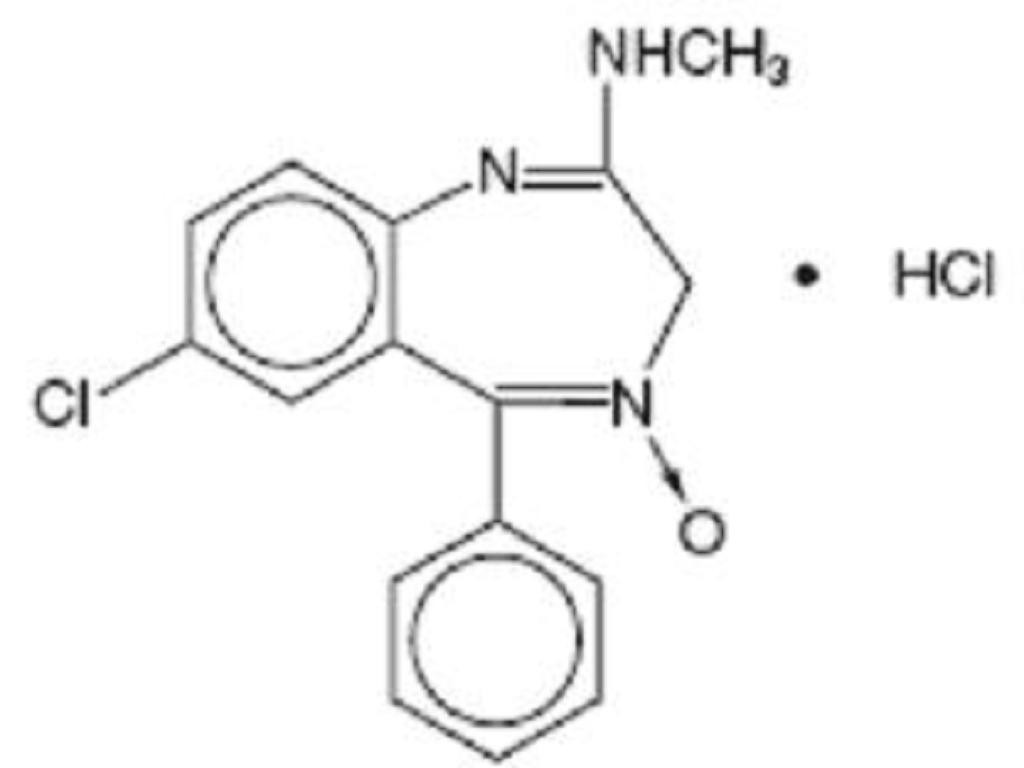
CLINICAL PHARMACOLOGY
Animal Pharmacology
Effects on Reproduction
INDICATIONS & USAGE
CHLORDIAZEPOXIDE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Usage in Pregnancy
An increased risk of congenital malformations associated with the use of minor tranquilizers (chlordiazepoxide, diazepam and meprobamate)during the first trimester of pregnancy has been suggested in several studies. Because use of these drugs is rarely a matter of urgency, their use during this period should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physicians about the desirability of discontinuing the drug.
DRUG ABUSE AND DEPENDENCE
PRECAUTIONS
PEDIATRIC USE
DOSAGE AND ADMINISTRATIONPRECAUTIONSINFORMATION FOR PATIENTS
CHLORDIAZEPOXIDE HYDROCHLORIDE ADVERSE REACTIONS
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
CONTRAINDICATIONSWARNINGSPRECAUTIONS
DOSAGE & ADMINISTRATION
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Chlordiazepoxide HydrochlorideChlordiazepoxide Hydrochloride CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!