Childrens Multi-Symptom Cold
Chain Drug Consortium, LLC
AptaPharma Inc.
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose
- Keep out of reach of children
- Childrens Multi-Symptom Cold Uses
- Warnings
- Ask a doctor before use
- When using this product
- Stop use and ask a doctor
- Directions
- Childrens Multi-Symptom Cold Other information
- Inactive ingredients
- Questions?
- Product Label
FULL PRESCRIBING INFORMATION
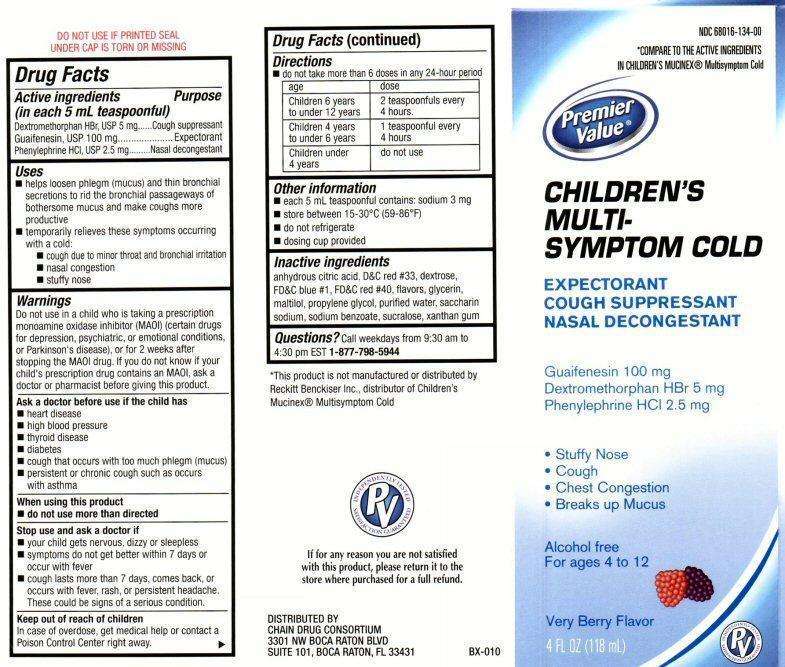
Active ingredients
Drug Facts
Active ingredients
(in each 5 mL teaspoon)
Guaifenesin 100 mg
Dextromethorphan HBr 5 mg
Phenylephrine HCL 2.5 mg
Purpose
Purpose
Cough suppressant
Expectorant
Nasal decongestant
Keep out of reach of children
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control center right away.
Childrens Multi-Symptom Cold Uses
Uses
- helps to loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passage ways of bothersome mucus and make coughs more productive
- temporarily relieves these symptoms occurring with a cold:
- cough due to minor throat and bronchial irritation
- nasal congestion
- stuffy nose
Warnings
Warnings
Do not use in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for two weeks after stopping the MAOI drug. If you do not know if your child’s prescription drug contains an MAOI, ask a doctor or a pharmacist before taking this product.
Ask a doctor before use
Ask a doctor before use if the child has
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with asthma
When using this product
When using this product
- do not use more than directed
Stop use and ask a doctor
Stop use and ask a doctor if
- your child gets nervous, dizzy, or sleepless
- symptoms do not get better within 7 days or occur with fever
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache.
These could be signs of a serious condition.
Directions
Directions
- do not take more than 6 doses in any 24-hour period
Age Dose
Children 6 years to under 12 years 2 teaspoons every 4 hours
Children 4 years to under 6 years 1 teaspoon every 4 hours
Children under 4 years Do not use
Childrens Multi-Symptom Cold Other information
Other information
- each 5 mL teaspoon contains: sodium 3 mg
- store between 15-30 ° C (59-86° F)
- do not refrigerate
- dosing cup provided
Inactive ingredients
Inactive ingredients
citric acid anhydrous, D and C red #33,dextrose, FD and C blue #1, FD and C red # 40, flavors, glycerin, maltitol, propylene glycol, purified water, saccharin sodium, sodium benzoate, sucralose, xanthan gum
Questions?
Questions?
Call weekdays from 9:30 AM to 4:30 PM EST at
1-877-798-5944
Product Label
NDC 68016-134-00
*COMPARE TO THE ACTIVE INGREDIENTS IN CHILDREN’S MUCINEX®Multisymptom Cold
Premier Value®
CHILDREN’S MULTI- SYMPTOM COLD
EXPECTORANT
COUGH SUPPRESSANT
NASAL DECONGESTANT
Guaifenesin 100 mg
Dextromethorphan HBr 5 mg
Phenylephrine HCL 2.5 mg
* Stuffy Nose
* Cough
* Chest Congestion
* Breaks up Mucus
Alcohol free
For ages 4 to 12
Very Berry Flavor
4 FL OZ (118mL)
INDEPENDENTLY TESTED SATISFACTION GUARANTEED PV
DO NOT USE IF PRINTED SEAL UNDER CAP IS TORN OR MISSING
*This product is not manufactured or distributed by Reckitt Benckiser Inc. distributor of Children’s Mucinex® Multisymptom Cold
If for Any reason you are not satisfied with this product, lease return it to the store where purchased for a full refund.
DISTRIBUTED BY
CHAIN DRUG CONSORTIUM
3301 NW BOCA RATON BLVD
SUITE 101, BOCA RATON, FL 33431
BX-010
Childrens Multi-Symptom ColdGUAIFENESIN, DEXTROMETHORPHAN HYDROBROMIDE, PHENYLEPHRINE HYDROCHLORIDE LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||