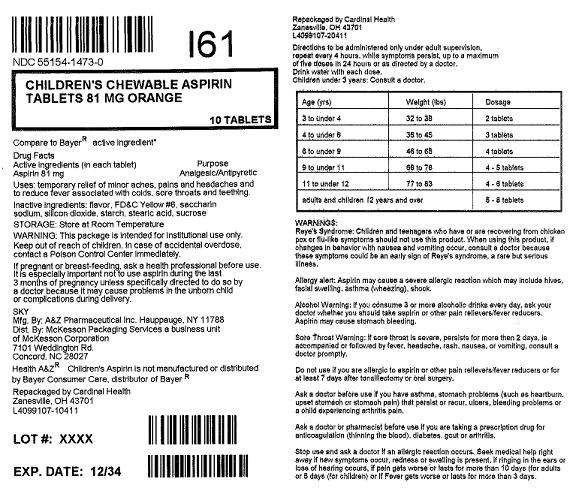
Childrens Chewable Aspirin
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Childrens Chewable Aspirin Uses
- Warnings
- Directions
- Inactive ingredients
- Storage
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Aspirin 81 mg
Purpose
Analgesic/Antipyretic
Keep Out of Reach of Children
Keep out of the reach of children.
Childrens Chewable Aspirin Uses
Temporary relief of minor aches, pains and headaches and to reduce fever associated with colds, sore throats and teething.
Warnings
This package is intended for institutional use only. In case of accidental overdose, contact a Poison Control Center Immediately.
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless specifically directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Reye's Syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's Syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include hives, facial swelling, asthma (wheezing), shock.
Alcohol Warning: If you consume 3 or more alcoholic drinks everyday, ask your doctor whether you should take aspirin or other pain relievers/fever reducers.
Aspirin may cause stomach bleeding.
Sore Throat Warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use if you are allergic to aspirin or other pain relievers/fever reducers or for at least 7 days after tonsillectomy or oral surgery.
Directions
To be administered only under adult supervision, repeat every 4 hours, while symptoms persist, up to a maximum of five doses in 24 hours or as directed by a doctor.
Drink water with each dose.
Children under 3 years: Consult a doctor.
| Age (yrs) | Weight (lbs) | Dosage |
| 3 to under 4 | 32 to 35 | 2 tablets |
| 4 to under 6 | 35 to 45 | 3 tablets |
| 6 to under 9 | 46 to 65 | 4 tablets |
| 9 to under 11 | 66 to 76 | 4-5 tablets |
| 11 to under 12 | 77 to 83 | 4-6 tablets |
| adults and children 12 years and over | 5-8 tablets | |
Ask a doctor before use if you have asthma, stomach problems (such as heartburn, upset stomach or stomach pain) that persist or recur, ulcers, bleeding problems or a child experiencing arthritis pain.
Ask a doctor or pharmacist before use if you are taking a prescription drug for anticoagulation (thinning the blood), diabetes, gout or arthritis.
Stop use and ask a doctor if an allergic reaction occurs. Seek medical help right away if new symptoms occur, redness or swelling is present. If ringing in the ears or loss of hearing occurs, if pain gets worse or lasts for more than 10 days (for adults) or 5 days (for children) or if fever gets worse or lasts for more than 3 days.
Inactive ingredients
flavor, FD&C Yellow #6, saccharin sodium, silicon dioxide, starch, stearic acid, sucrose
Storage
Store at Room Temperature
SKY
MFG. BY: A&Z Pharmaceutical Inc. Hauppauge, NY 11788
Dist. BY: McKesson Packaging Services a business unit of McKesson Corporation
7101 Weddington Rd.
Concord, NC 28027
Health A&Z® Children's Aspirin is not manufactured or distributed by Bayer Consumer Care, distributor of Bayer®
Repackaged by Cardinal Health
Zanesville, OH 43701
Principal Display Panel
Children's Chewable Aspirin Orange
81 mg
10 Tablets
Childrens Chewable AspirinAspirin TABLET, CHEWABLE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||