Childrens Allergy Relief
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- Childrens Allergy Relief Uses
- Warnings
- Directions
- Childrens Allergy Relief Other information
- Inactive ingredients
- Questions?
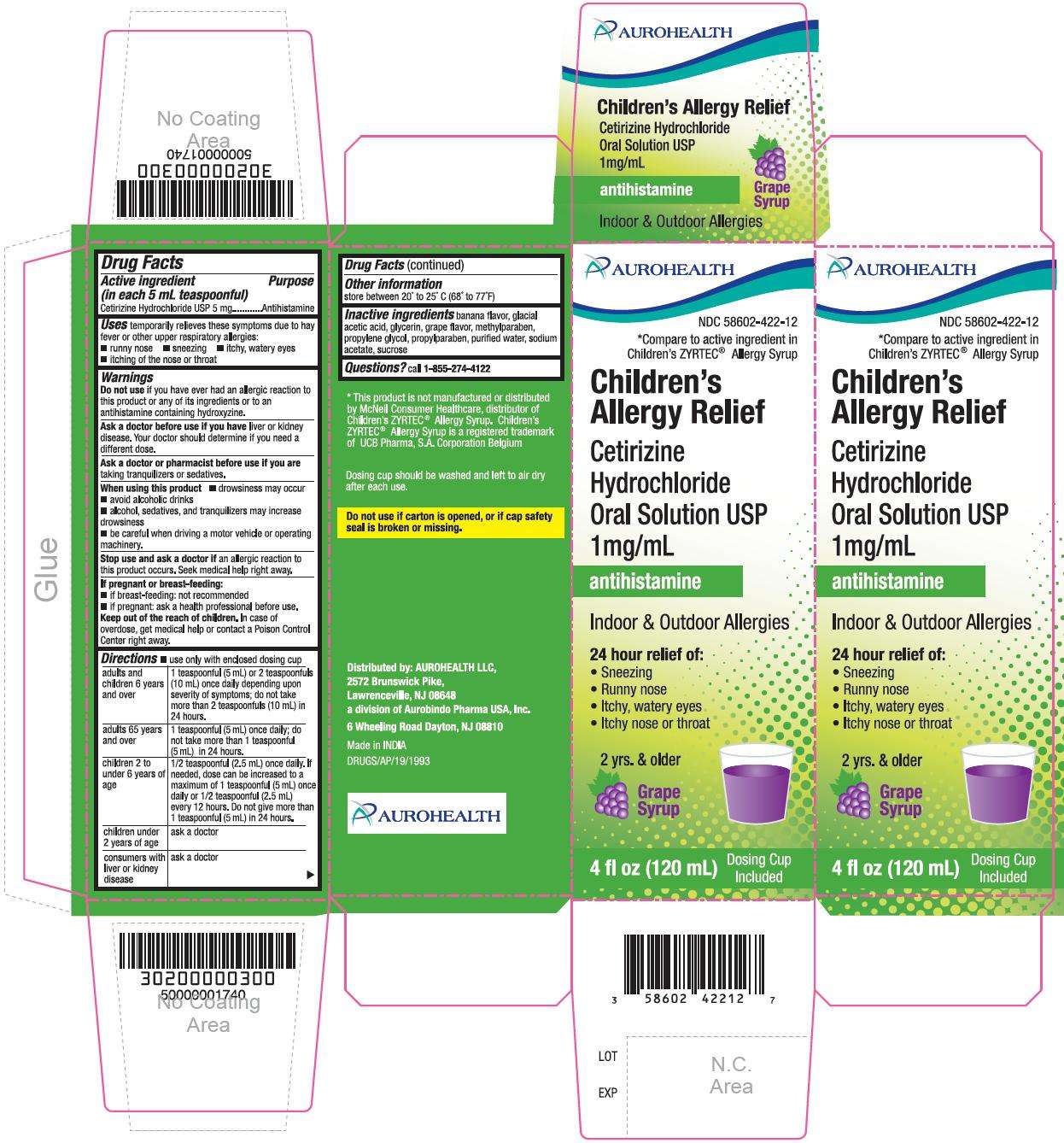
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 mg/mL (120 mL Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 mg/mL Carton (120 mL)
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient (in each 5 mL teaspoonful)
Cetirizine Hydrochloride USP 5 mg
Purpose
Antihistamine
Childrens Allergy Relief Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
Ask a doctor or pharmacist before use if you are
taking tranquilizers or sedatives.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery.
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding:
- If breast-feeding: not recommended
- If pregnant: ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- use only with enclosed dosing cup
| adults and children 6 years and over |
1 teaspoonful (5 mL) or 2 teaspoonfuls (10 mL) once daily depending upon severity of symptoms; do not take more than 2 teaspoonfuls (10 mL) in 24 hours. |
| adults 65 years and over |
1 teaspoonful (5 mL) once daily; do not take more than 1 teaspoonful (5 mL) in 24 hours. |
| children 2 to under 6 years of age |
½ teaspoonful (2.5 mL) once daily. If needed, dose can be increased to a maximum of 1 teaspoonful (5 mL) once daily or ½ teaspoonful (2.5 mL) every 12 hours. Do not give more than 1 teaspoonful (5 mL) in 24 hours. |
| children under 2 years of age |
ask a doctor |
| consumers with liver or kidney disease |
ask a doctor |
Childrens Allergy Relief Other information
- store between 20° to 25°C (68° to 77°F)
Inactive ingredients
banana flavor, glacial acetic acid, glycerin, grape flavor, methylparaben, propylene glycol, propylparaben, purified water, sodium acetate, sucrose
Questions?
call 1-855-274-4122
*This product is not manufactured or distributed by McNeil Consumer Healthcare, distributor of Children's ZYRTEC® Allergy Syrup. Children's ZYRTEC® Allergy Syrup is a registered trademark of UCB Pharma, S.A. Corporation Belgium
Dosing cup should be washed and left to air dry after each use.
Do not use if carton is opened, or if cap safety seal is broken or missing.
Distributed by: AUROHEALTH LLC,
2572 Brunswick Pike,
Lawrenceville, NJ 08648
a division of Aurobindo Pharma USA, Inc.
6 Wheeling Road, Dayton NJ 08810
Made in INDIA
DRUGS/AP/19/1993
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 mg/mL (120 mL Bottle)
AUROHEALTH
NDC 58602-422-12
*Compare to active ingredient in
Children's ZYRTEC®Allergy Syrup
Children's Allergy Relief
Cetirizine Hydrochloride
Oral Solution USP
1mg/mL
antihistamine
Indoor & Outdoor Allergies
24 hour
relief
of:
- Sneezing
- Runny nose
- Itchy, watery eyes
- Itchy nose or throat
Grape Syrup
4 fl oz (120 mL) 2 yrs. & older

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 mg/mL Carton (120 mL)
AUROHEALTH
NDC 58602-422-12
*Compare to active ingredient in
Children’s ZYRTEC® Allergy Syrup
Children's
Allergy Relief
Cetirizine
Hydrochloride
Oral Solution USP
1mg/mL
antihistamine
Indoor & Outdoor Allergies
24 hour
Relief of
- Sneezing
- Runny nose
- Itchy, watery eyes
- Itchy nose or throat
2 yrs.
& older
Grape
Syrup
4 fl oz (120 mL) Dosing Cup
Included
Childrens Allergy ReliefCetirizine Hydrochloride SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||