CETIRIZINE HYDROCHLORIDE
CETIRIZINE HYDROCHLORIDE SYRUP For Oral Use Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- CETIRIZINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CETIRIZINE HYDROCHLORIDE INDICATIONS AND USAGE
- CETIRIZINE HYDROCHLORIDE CONTRAINDICATIONS
- PRECAUTIONS
- CETIRIZINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- CETIRIZINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
CETIRIZINE HYDROCHLORIDE DESCRIPTION
1212523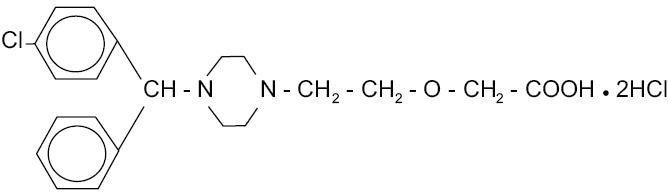
CLINICAL PHARMACOLOGY
Mechanism of Actions
1In vivoex vivo In vitro1 Ex vivo1Pharmacokinetics
Absorption
maxmaxmaxmax
Distribution
Metabolism
Elimination
Interaction Studies
Special Populations
Pediatric Patients
maxmax(0-t)
Effect of Gender
Effect of Race
Pharmacodynamics
Clinical Studies
CETIRIZINE HYDROCHLORIDE INDICATIONS AND USAGE
Perennial Allergic Rhinitis
Chronic Urticaria
CETIRIZINE HYDROCHLORIDE CONTRAINDICATIONS
PRECAUTIONS
Activities Requiring Mental Alertness
Drug-Drug Interactions
Carcinogenesis, Mutagenesis and Impairment of Fertility
2 2 2222in vivo
2
Pediatric Use
max
CETIRIZINE HYDROCHLORIDE ADVERSE REACTIONS
| Adverse Experiences | Placebo (N=309) |
Cetirizine hydrochloride | |
|---|---|---|---|
| 5 mg (N=161) |
10 mg (N=215) |
||
| Headache |
12.3% |
11.0% |
14.0% |
| Pharyngitis |
2.9% |
6.2% |
2.8% |
| Abdominal pain |
1.9% |
4.4% |
5.6% |
| Coughing |
3.9% |
4.4% |
2.8% |
| Somnolence |
1.3% |
1.9% |
4.2% |
| Diarrhea |
1.3% |
3.1% |
1.9% |
| Epistaxis |
2.9% |
3.7% |
1.9% |
| Bronchospasm |
1.9% |
3.1% |
1.9% |
| Nausea |
1.9% |
1.9% |
2.8% |
| Vomiting |
1.0% |
2.5% |
2.3% |
Autonomic Nervous System
Cardiovascular
Central and Peripheral Nervous Systems
Gastrointestinal
Genitourinary
Hearing and Vestibular
Metabolic/Nutritional
Musculoskeletal
Psychiatric
Respiratory System
Reproductive
Reticuloendothelial
Skin
Special Senses
Vision
Body as a Whole
Occasional instances of transient, reversible hepatic transaminase elevations have occurred during cetirizine therapy. Hepatitis with significant transaminase elevation and elevated bilirubin in association with the use of cetirizine hydrochloride has been reported.
Post-Marketing Experience
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
2222CETIRIZINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Children 2 to 5 Years for Chronic Urticaria
Children 6 months to <2 years for Perennial Allergic Rhinitis and Chronic Uriticaria
HOW SUPPLIED
STORAGE: Store at 20-25°C (68-77°F)or Store refrigerated, 2-8°C (36-46°F).
Manufactured by:
Distributed by:

CETIRIZINE HYDROCHLORIDECETIRIZINE HYDROCHLORIDE SYRUP
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!