Cetirizine Hydrochloride
Cypress Pharmaceutical, Inc.
Cypress Pharmaceutical, Inc.
Cetirizine Hydrochloride Syrup
FULL PRESCRIBING INFORMATION: CONTENTS*
- CLINICAL PHARMACOLOGY
- CETIRIZINE HYDROCHLORIDE INDICATIONS AND USAGE
- CETIRIZINE HYDROCHLORIDE CONTRAINDICATIONS
- PRECAUTIONS
- CETIRIZINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- CETIRIZINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Product Packaging
- Product Packaging
FULL PRESCRIBING INFORMATION
For Oral UseRx only
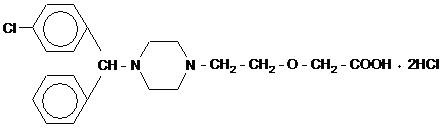
DESCRIPTION
1212523
CLINICAL PHARMACOLOGY
Mechanism of Actions:1In vivoex vivo In vitro1Ex vivo1
Pharmacokinetics:
Absorption:maxmaxmax maxDistribution:
Metabolism:
Elimination:
Interaction Studies
Special Populations
Pediatric Patients:max max(0-t)Effect of Gender:
Effect of Race:
Pharmacodynamics:
CETIRIZINE HYDROCHLORIDE INDICATIONS AND USAGE
Perennial Allergic Rhinitis:Chronic Urticaria:
CETIRIZINE HYDROCHLORIDE CONTRAINDICATIONS
PRECAUTIONS
Activities Requiring Mental Alertness:Drug-Drug Interactions:
Carcinogenesis, Mutagenesis and Impairment of Fertility222222
in vivo
2
Pediatric Use:
max
CETIRIZINE HYDROCHLORIDE ADVERSE REACTIONS
Pediatric studies were also conducted with cetirizine hydrochloride. More than 1300 pediatric patients aged 6 to 11 years with more than 900 treated with cetirizine hydrochloride at doses of 1.25 to 10 mg per day were included in controlled and uncontrolled clinical trials conducted in the United States. The duration of treatment ranged from 2 to 12 weeks. Placebo-controlled trials up to 4 weeks duration included 168 pediatric patients aged 2 to 5 years who received cetirizine, the majority of whom received single daily doses of 5 mg. A placebo-controlled trial 18 months in duration included 399 patients aged 12 to 24 months treated with cetirizine (0.25 mg/kg bid), and another placebo-controlled trial of 7 days duration included 42 patients aged 6 to 11 months who were treated with cetirizine (0.25 mg/kg bid).
The majority of adverse reactions reported in pediatric patients aged 2 to 11 years with cetirizine hydrochloride were mild or moderate. In placebo-controlled trials, the incidence of discontinuations due to adverse reactions in pediatric patients receiving up to 10 mg of cetirizine hydrochloride was uncommon (0.4% on cetirizine hydrochloride vs. 1.0% on placebo).
Table 1 lists adverse experiences which were reported for cetirizine hydrochloride 5 and 10 mg in pediatric patients aged 6 to 11 years in placebo-controlled clinical trials in the United States and were more common with cetirizine hydrochloride than placebo. Of these, abdominal pain was considered treatment-related and somnolence appeared to be dose-related, 1.3% in placebo, 1.9% at 5 mg and 4.2% at 10 mg. The adverse experiences reported in pediatric patients aged 2 to 5 years in placebo-controlled trials were qualitatively similar in nature and generally similar in frequency to those reported in trials with children aged 6 to 11 years.
In the placebo-controlled trials of pediatric patients 6 to 24 months of age, the incidences of adverse experiences were similar in the cetirizine and placebo treatment groups in each study. Somnolence occurred with essentially the same frequency in patients who received cetirizine and patients who received placebo. In a study of 1 week duration in children 6-11 months of age, patients who received cetirizine exhibited greater irritability/fussiness than patients on placebo. In a study of 18 months duration in patients 12 months and older, insomnia occurred more frequently in patients who received cetirizine compared to patients who received placebo (9.0% v. 5.3%). In those patients who received 5 mg or more per day of cetirizine as compared to patients who received placebo, fatigue (3.6% v. 1.3%) and malaise (3.6% v. 1.8%) occurred more frequently.

The following events were observed infrequently (less than 2%), in either 3982 adults and children 12 years and older or in 659 pediatric patients aged 6 to 11 years who received cetirizine hydrochloride in U.S. trials, including an open adult study of six months duration. A causal relationship of these infrequent events with cetirizine hydrochloride administration has not been established.
Autonomic Nervous System: anorexia, flushing, increased salivation, urinary retention.
Cardiovascular: cardiac failure, hypertension, palpitation, tachycardia.
Central and Peripheral Nervous Systems: abnormal coordination, ataxia, confusion, dysphonia, hyperesthesia, hyperkinesia, hypertonia, hypoesthesia, leg cramps, migraine, myelitis, paralysis, paresthesia, ptosis, syncope, tremor, twitching, vertigo, visual field defect.
Gastrointestinal: abnormal hepatic function, aggravated tooth caries, constipation, dyspepsia, eructation, flatulence, gastritis, hemorrhoids, increased appetite, melena, rectal hemorrhage, stomatitis including ulcerative stomatitis, tongue discoloration, tongue edema.
Genitourinary: cystitis, dysuria, hematuria, micturition frequency, polyuria, urinary incontinence, urinary tract infection.
Hearing and Vestibular: deafness, earache, ototoxicity, tinnitus.
Metabolic/Nutritional: dehydration, diabetes mellitus, thirst.
Musculoskeletal: arthralgia, arthritis, arthrosis, muscle weakness, myalgia.
Psychiatric: abnormal thinking, agitation, amnesia, anxiety, decreased libido, depersonalization, depression, emotional lability, euphoria, impaired concentration, insomnia, nervousness, paroniria, sleep disorder.
Respiratory System: bronchitis, dyspnea, hyperventilation, increased sputum, pneumonia, respiratory disorder, rhinitis, sinusitis, upper respiratory tract infection.
Reproductive: dysmenorrhea, female breast pain, intermenstrual bleeding, leukorrhea, menorrhagia, vaginitis.
Reticuloendothelial: lymphadenopathy.
Skin: acne, alopecia, angioedema, bullous eruption, dermatitis, dry skin, eczema, erythematous rash, furunculosis, hyperkeratosis, hypertrichosis, increased sweating, maculopapular rash, photosensitivity reaction, photosensitivity toxic reaction, pruritus, purpura, rash, seborrhea, skin disorder, skin nodule, urticaria.
Special Senses: parosmia, taste loss, taste perversion.
Vision: blindness, conjunctivitis, eye pain, glaucoma, loss of accommodation, ocular hemorrhage, xerophthalmia.
Body as a Whole: accidental injury, asthenia, back pain, chest pain, enlarged abdomen, face edema, fever, generalized edema, hot flashes, increased weight, leg edema, malaise, nasal polyp, pain, pallor, periorbital edema, peripheral edema, rigors.
Occasional instances of transient, reversible hepatic transaminase elevations have occurred during cetirizine therapy. Hepatitis with significant transaminase elevation and elevated bilirubin in association with the use of cetirizine hydrochloride has been reported.
Post-Marketing Experience
In the post-marketing period, the following additional rare, but potentially severe adverse events have been reported: aggressive reaction, anaphylaxis, cholestasis, convulsions, glomerulonephritis, hallucinations, hemolytic anemia, hepatitis, orofacial dyskinesia, severe hypotension, stillbirth, suicidal ideation, suicide and thrombocytopenia.
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
2222CETIRIZINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Children 2 to 5 Years for Chronic Urticaria:
Children 6 months to less than 2 years for Perennial Allergic Rhinitis and Chronic Uriticaria:
HOW SUPPLIED
STORAGE: Store at 20-25°C (68°-77°F);or Store refrigerated, 2-8°C (36-46°F).
Rx Only
Product Packaging
NDC 60258-860-16
Cetirizine
Hydrochloride
Syrup,
1 mg/1 mL*
Rx Only
1 pint (480 mL)
CYPRESS
PHARMACEUTICAL, INC.
Store at 20°-25°C (68°-77°F);
excursions permitted to 15°-30°C
(59°-86°F)
or Store
refrigerated, 2°-8°C (36°-46°F).
DOSAGE AND USE

Product Packaging
NDC 60258-860-04
Cetirizine
Hydrochloride
Syrup,
1 mg/1 mL*
Rx Only
CYPRESS
PHARMACEUTICAL, INC.
120 mL
Store at 20°-25°c (68°-77°F);
excursions permitted to 15°-30°C
(59°-86°F)
or Store refrigerated,
2°-8°C (36°-46°F).
DOSAGE AND USE

Cetirizine HydrochlorideCetirizine Hydrochloride SYRUP
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||