Cetacaine Topical Anesthetic
Cetacaine Topical Anesthetic GEL
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
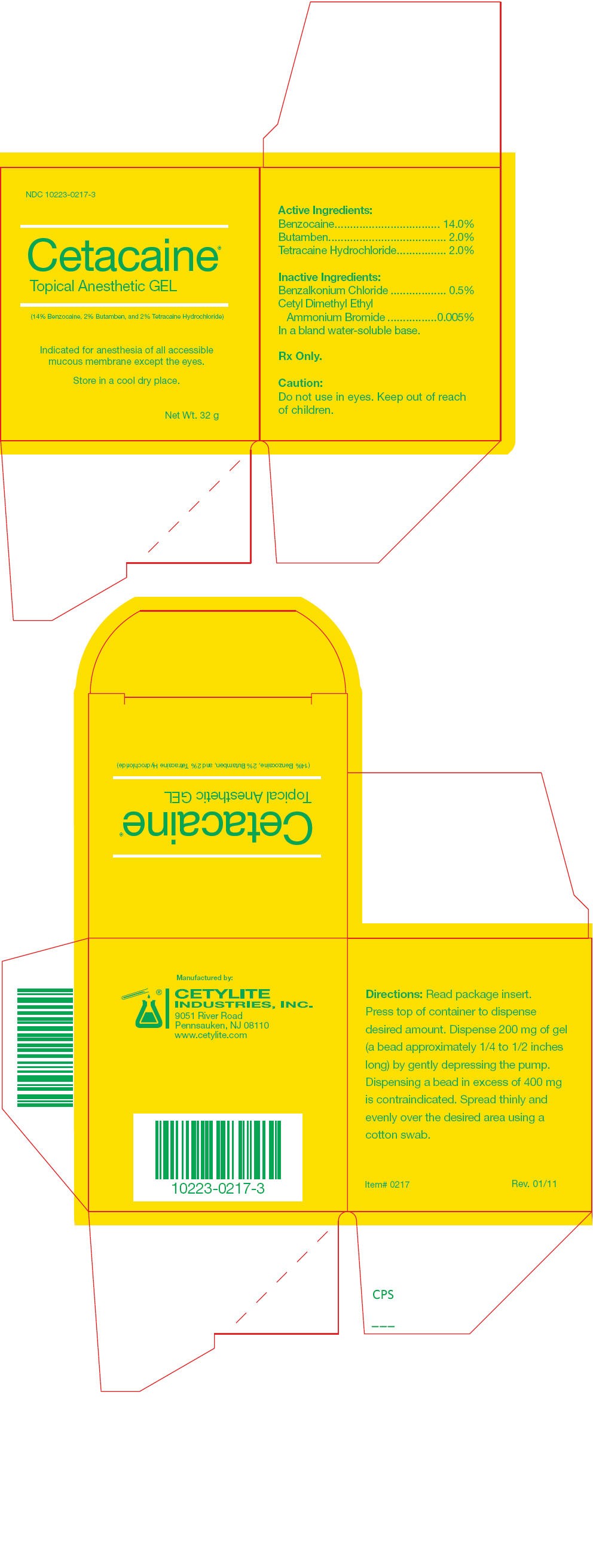
| Active Ingredients: | |
|---|---|
| Benzocaine | 14.0% |
| Butamben | 2.0% |
| Tetracaine Hydrochloride | 2.0% |
| Inactive Ingredients: | |
|---|---|
| Benzalkonium Chloride | 0.5% |
| Cetyl Dimethyl Ethyl | |
| Ammonium Bromide | 0.005% |
| In a bland water-soluble base. | |
Rx Only.
Caution
Do not use in eyes. Keep out of reach of children.
Directions
Read package insert. Press top of container to dispense desired amount. Dispense 200 mg of gel (a bead approximately 1/4 to 1/2 inches long) by gently depressing the pump. Dispensing a bead in excess of 400 mg is contraindicated. Spread thinly and evenly over the desired area using a cotton swab.
Item# 0217
Rev. 01/11
Manufactured by:
CETYLITE INDUSTRIES, INC.
9051 River Road
Pennsauken, NJ 08110
www.cetylite.com
PRINCIPAL DISPLAY PANEL - 32 g Jar Box
NDC 10223-0217-3
Cetacaine
®
Topical Anesthetic GEL
(14% Benzocaine, 2% Butamben, and 2% Tetracaine Hydrochloride)
Indicated for anesthesia of all accessible
mucous membrane except the eyes.
Store in a cool dry place.
Net Wt. 32 g
Cetacaine Topical AnestheticBenzocaine, Butamben, and Tetracaine Hydrochloride GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||