Cephalexin
Cephalexin Capsules, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- Rx only
- CEPHALEXIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE SECTION
- CEPHALEXIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CEPHALEXIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION SECTION
- HOW SUPPLIED
- REFERENCES
FULL PRESCRIBING INFORMATION
Rx only
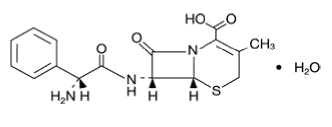
CEPHALEXIN DESCRIPTION
161734•2
D
CLINICAL PHARMACOLOGY
Human Pharmacology
Microbiology
In vitro in vitro INDICATIONS AND USAGE
Aerobes, Gram-positive
Staphylococcus aureus
Streptococcus pneumoniae
Streptococcus pyogenes
Aerobes, Gram-negative
Escherichia coli
Haemophilus influenzae
Klebsiella pneumoniae
Moraxella (Branhamella) catarrhalis
Proteus mirabilis
Note —Enterococcus faecalisStreptococcus faecalisEnterobacterMorganella morganiiProteus vulgarisPseudomonasAcinetobacter calcoaceticusStreptococcus pneumoniae
Dilution techniques — 1 to 3
|
MIC (mcg/mL) |
Interpretation |
|
≤8 |
Susceptible (S) |
|
16 |
Intermediate (I) |
|
≥32 |
Resistant (R) |
|
Microorganism |
MIC (mcg/mL) |
|
E. coli ATCC 25922 |
4-16 |
|
S. aureus ATCC 29213 |
0.12-0.5 |
|
Zone Diameter (mm) |
Interpretation |
|
≥18 |
Susceptible (S) |
|
15-17 |
Intermediate (I) |
|
≤14 |
Resistant (R) |
|
Microorganism |
Zone Diameter (mm) |
|
E. coli ATCC 25922 |
15-21 |
|
S. aureus ATCC 25923 |
29-37 |
INDICATIONS & USAGE SECTION
Streptococcus pneumoniaeStreptococcus pyogenes
Streptococcus pneumoniaeHaemophilus influenzaeStaphylococcus aureus, Streptococcus pyogenes,Moraxella catarrhalis
Staphylococcus aureusStreptococcus pyogenes
Staphylococcus aureus Proteus mirabilis
Escherichia coliProteus mirabilis, Klebsiella pneumoniae
Note —
CEPHALEXIN CONTRAINDICATIONS
WARNINGS
BEFORE THERAPY WITH CEPHALEXIN IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEPHALEXIN, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEPHALEXIN OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile C. difficile
C. difficile C. difficile
C. difficileC. difficile
PRECAUTIONS
General
Information for Patients
Drug Interactions
Metformin max
Probenecid
Drug & Or Laboratory Test Interactions Section
As a result of administration of cephalexin, a false-positive reaction for glucose in the urine may occur. This has been observed with Benedict's and Fehling's solutions and also with Clinitest® tablets.
Carcinogenesis, Mutagenesis, Impairment of Fertility
2
Pregnancy
Teratogenic Effects
Pregnancy Category B —2
Nursing Mothers
Pediatric Use
DOSAGE AND ADMINISTRATION
Geriatric Use
PRECAUTIONS, General
CEPHALEXIN ADVERSE REACTIONS
Gastrointestinal — WARNINGS
Hypersensitivity —
Adverse Reactions
INDICATIONS AND USAGE PRECAUTIONS, General
Altered Laboratory Tests
OVERDOSAGE
Signs and Symptoms —
Treatment — Physicians' Desk Reference (PDR).
DOSAGE & ADMINISTRATION SECTION
Adults —
Pediatric Patients —
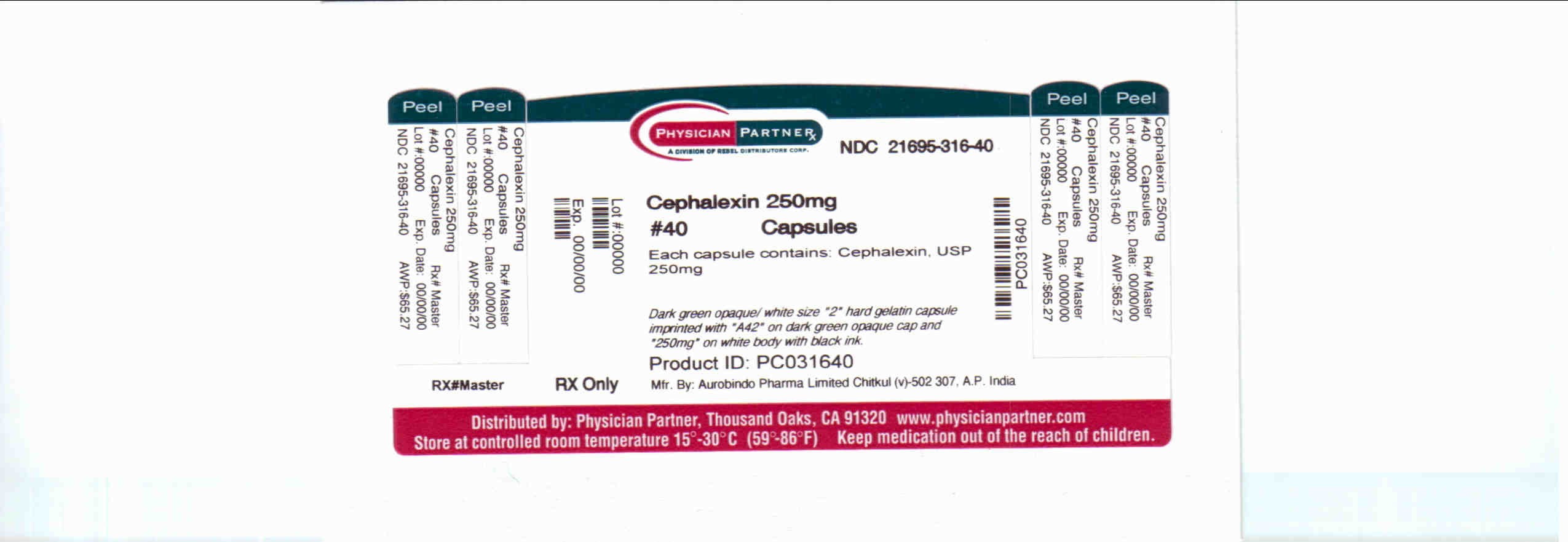
HOW SUPPLIED
250 mg Capsule
500 mg Capsule
Store at
REFERENCES
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically — Fourth Edition. Approved Standard NCCLS Document M7-A4, Vol. 17, No. 2, NCCLS, Wayne, PA, January, 1997.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests — Sixth Edition. Approved Standard NCCLS Document M2-A6, Vol. 17, No. 1, NCCLS, Wayne, PA, January, 1997.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing — Eighth Informational Supplement. Approved Standard NCCLS Document M100-S8, Vol. 18, No. 1, NCCLS, Wayne, PA, January, 1998.
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
Rebel Distributors Corp.
CephalexinCephalexin CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CephalexinCephalexin CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!