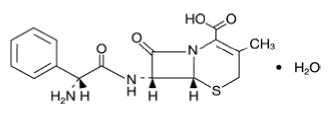
CEPHALEXIN
FULL PRESCRIBING INFORMATION
161734•2
 LABEL IMAGE FOR 250MG
LABEL IMAGE FOR 250MG
 LABEL IMAGE FOR 500MG
LABEL IMAGE FOR 500MG
In vitro in vitro INDICATIONS AND USAGE
Aerobes, Gram-positive
Staphylococcus aureus
Streptococcus pneumoniae
Streptococcus pyogenes
Aerobes, Gram-negative
Escherichia coli
Haemophilus influenzae
Klebsiella pneumoniae
Moraxella (Branhamella) catarrhalis
Proteus mirabilis
Note —Enterococcus faecalisStreptococcus faecalisEnterobacterMorganella morganiiProteus vulgarisPseudomonasAcinetobacter calcoaceticusStreptococcus pneumoniae
Dilution techniques — 1 to 3
Diffusion techniques — 2,3
Uses
Streptococcus pneumoniaeStreptococcus pyogenes
Streptococcus pneumoniaeHaemophilus influenzaeStaphylococcus aureus, Streptococcus pyogenes,Moraxella catarrhalis
Staphylococcus aureusStreptococcus pyogenes
Staphylococcus aureus Proteus mirabilis
Escherichia coliProteus mirabilis, Klebsiella pneumoniae
Note —
BEFORE THERAPY WITH CEPHALEXIN IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEPHALEXIN, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEPHALEXIN OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile C. difficile
C. difficile C. difficile
C. difficileC. difficile
Metformin max
Probenecid
As a result of administration of cephalexin, a false-positive reaction for glucose in the urine may occur. This has been observed with Benedict's and Fehling's solutions and also with Clinitest® tablets.
2
Pregnancy Category B —2
DOSAGE AND ADMINISTRATION
PRECAUTIONS, General
Gastrointestinal — WARNINGS
Hypersensitivity —
Adverse Reactions
INDICATIONS AND USAGE PRECAUTIONS, General
Altered Laboratory Tests
Signs and Symptoms —
Treatment — Physicians' Desk Reference (PDR).
Adults —
Pediatric Patients —
HOW SUPPLIED
Cephalexin Capsules, USP are available in:
250 mg Capsule
Dark green opaque/white size “2” hard gelatin capsule filled with off
white granular powder and imprinted with “A 42” on dark green opaque cap and
“250 mg” on white body with black ink.
Bottles of
20 NDC
65862-018-20
Bottles of
40 NDC
65862-018-40
Bottles of
100 NDC
65862-018-01
Bottles of
500 NDC 65862-018-05
500 mg Capsule
Dark green opaque/light green opaque size “0” hard gelatin capsule
filled with off white granular powder and imprinted with “A 43” on dark green
opaque cap and “500 mg” on light green opaque body with black
ink.
Bottles of 20
NDC 65862-019-20
Bottles of
40 NDC
65862-019-40
Bottles of 100
NDC 65862-019-01
Bottles of
500 NDC 65862-019-05
Store at 20° to 25°C (68° to 77°F); excursions permitted to
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
CEPHALEXINCEPHALEXIN CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CEPHALEXINCEPHALEXIN CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||