Cefepime
Cefepime for Injection, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- CEFEPIME DESCRIPTION
- CLINICAL PHARMACOLOGY
- CEFEPIME INDICATIONS AND USAGE
- CLINICAL STUDIES
- CEFEPIME CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CEFEPIME ADVERSE REACTIONS
- OVERDOSAGE
- CEFEPIME DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
FULL PRESCRIBING INFORMATION
For Intravenous or Intramuscular Use
Rx Only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefepime for injection, USP and other antibacterial drugs, cefepime for injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
CEFEPIME DESCRIPTION
Cefepime for injection, USP is a semi-synthetic, broad spectrum, cephalosporin antibiotic for parenteral administration. The chemical name is 1-[[(6R,7R)-7-[2-(2-amino-4-thiazolyl)-glyoxylamido]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl] methyl]-1-methylpyrrolidinium chloride, 72-(Z)-(O-methyloxime), monohydrochloride, monohydrate, which corresponds to the following structural formula:

Cefepime hydrochloride is a white to pale yellow powder. Cefepime hydrochloride contains the equivalent of not less than 825 mcg and not more than 911 mcg of cefepime (C19H24N6O5S2) per mg, calculated on an anhydrous basis. It is highly soluble in water.
Cefepime for injection, USP is supplied for intramuscular or intravenous administration in strengths equivalent to 500 mg, 1 g, and 2 g of cefepime. (See DOSAGE AND ADMINISTRATION .) Cefepime for injection, USP is a sterile, dry mixture of cefepime hydrochloride and L-arginine. It contains the equivalent of not less than 90 percent and not more than 115 percent of the labeled amount of cefepime (C19H24N6O5S2). The L-arginine, at an approximate concentration of 725 mg/g of cefepime, is added to control the pH of the constituted solution at 4 to 6. Freshly constituted solutions of cefepime for injection, USP will range in color from pale yellow to amber.
CLINICAL PHARMACOLOGY
Cefepime is an antibacterial agent belonging to the cephalosporin class of antibacterials with in vitro antibacterial activity against facultative Gram-positive and Gram-negative bacteria.
Pharmacokinetics
The average plasma concentrations of cefepime observed in healthy adult male volunteers (n=9) at various times following single 30 minute infusions (IV) of cefepime 500 mg, 1 g, and 2 g are summarized in Table 1 . Elimination of cefepime is principally via renal excretion with an average (±SD) half-life of 2 (±0.3) hours and total body clearance of 120 (±8) mL/min in healthy volunteers. Cefepime pharmacokinetics are linear over the range 250 mg to 2 g. There is no evidence of accumulation in healthy adult male volunteers (n=7) receiving clinically relevant doses for a period of 9 days.
Absorption
The average plasma concentrations of cefepime and its derived pharmacokinetic parameters after intravenous (IV) administration are portrayed in Table 1 .
|
Parameter |
CEFEPIME |
||
|
500 mg IV |
1 g IV |
2 g IV |
|
|
0.5 h |
38.2 |
78.7 |
163.1 |
|
1 h |
21.6 |
44.5 |
85.8 |
|
2 h |
11.6 |
24.3 |
44.8 |
|
4 h |
5 |
10.5 |
19.2 |
|
8 h |
1.4 |
2.4 |
3.9 |
|
12 h |
0.2 |
0.6 |
1.1 |
|
Cmax, mcg/mL |
39.1 (3.5) |
81.7 (5.1) |
163.9 (25.3) |
|
AUC, h•mcg/mL |
70.8 (6.7) |
148.5 (15.1) |
284.8 (30.6) |
|
Number of subjects |
9 |
9 |
9 |
Following intramuscular (IM) administration, cefepime is completely absorbed. The average plasma concentrations of cefepime at various times following a single intramuscular injection are summarized in Table 2 . The pharmacokinetics of cefepime are linear over the range of 500 mg to 2 g intramuscularly and do not vary with respect to treatment duration.
| Parameter | CEFEPIME | ||
|
500 mg IM |
1 g IM |
2 g IM |
|
|
0.5 h |
8.2 |
14.8 |
36.1 |
|
1 h |
12.5 |
25.9 |
49.9 |
|
2 h |
12 |
26.3 |
51.3 |
|
4 h |
6.9 |
16 |
31.5 |
|
8 h |
1.9 |
4.5 |
8.7 |
|
12 h |
0.7 |
1.4 |
2.3 |
|
Cmax, mcg/mL |
13.9 (3.4) |
29.6 (4.4) |
57.5 (9.5) |
|
Tmax, h |
1.4 (0.9) |
1.6 (0.4) |
1.5 (0.4) |
|
AUC, h•mcg/mL |
60 (8) |
137 (11) |
262 (23) |
|
Number of subjects |
6 |
6 |
12 |
Distribution
The average steady-state volume of distribution of cefepime is 18 (±2) L. The serum protein binding of cefepime is approximately 20% and is independent of its concentration in serum.
Cefepime is excreted in human milk. A nursing infant consuming approximately 1000 mL of human milk per day would receive approximately 0.5 mg of cefepime per day. (See PRECAUTIONS: Nursing Mothers. )
Concentrations of cefepime achieved in specific tissues and body fluids are listed in Table 3 .
|
Tissue or Fluid |
Dose/Route |
# of Patients |
Average Time of |
Average |
|
Blister Fluid |
2 g IV |
6 |
1.5 |
81.4 mcg/mL |
|
Bronchial Mucosa |
2 g IV |
20 |
4.8 |
24.1 mcg/g |
|
Sputum |
2 g IV |
5 |
4 |
7.4 mcg/mL |
|
Urine |
500 mg IV |
8 |
0 to 4 |
292 mcg/mL |
|
1 g IV |
12 |
0 to 4 |
926 mcg/mL |
|
|
2 g IV |
12 |
0 to 4 |
3120 mcg/mL |
|
|
Bile |
2 g IV |
26 |
9.4 |
17.8 mcg/mL |
|
Peritoneal Fluid |
2 g IV |
19 |
4.4 |
18.3 mcg/mL |
|
Appendix |
2 g IV |
31 |
5.7 |
5.2 mcg/g |
|
Gallbladder |
2 g IV |
38 |
8.9 |
11.9 mcg/g |
|
Prostate |
2 g IV |
5 |
1 |
31.5 mcg/g |
Data suggest that cefepime does cross the inflamed blood-brain barrier. The clinical relevance of these data is uncertain at this time.
Metabolism and Excretion
Cefepime is metabolized to N-methylpyrrolidine (NMP) which is rapidly converted to the N-oxide (NMP-N-oxide). Urinary recovery of unchanged cefepime accounts for approximately 85% of the administered dose. Less than 1% of the administered dose is recovered from urine as NMP, 6.8% as NMP-N-oxide, and 2.5% as an epimer of cefepime. Because renal excretion is a significant pathway of elimination, patients with renal dysfunction and patients undergoing hemodialysis require dosage adjustment. (See DOSAGE AND ADMINISTRATION .)
Specific Populations
Renal impairment: Cefepime pharmacokinetics have been investigated in patients with various degrees of renal impairment (n=30). The average half-life in patients requiring hemodialysis was 13.5 (±2.7) hours and in patients requiring continuous peritoneal dialysis was 19 (±2) hours. Cefepime total body clearance decreased proportionally with creatinine clearance in patients with abnormal renal function, which serves as the basis for dosage adjustment recommendations in this group of patients. (See DOSAGE AND ADMINISTRATION .)
Hepatic impairment: The pharmacokinetics of cefepime were unaltered in patients with hepatic impairment who received a single 1 g dose (n=11).
Geriatric patients: Cefepime pharmacokinetics have been investigated in elderly (65 years of age and older) men (n=12) and women (n=12) whose mean (SD) creatinine clearance was 74 (±15) mL/min. There appeared to be a decrease in cefepime total body clearance as a function of creatinine clearance. Therefore, dosage administration of cefepime in the elderly should be adjusted as appropriate if the patient’s creatinine clearance is 60 mL/min or less. (See DOSAGE AND ADMINISTRATION .)
Pediatric patients: Cefepime pharmacokinetics have been evaluated in pediatric patients from 2 months to 11 years of age following single and multiple doses on every 8 hours (n=29) and every 12 hours (n=13) schedules. Following a single intravenous dose, total body clearance and the steady-state volume of distribution averaged 3.3 (±1) mL/min/kg and 0.3 (±0.1) L/kg, respectively. The urinary recovery of unchanged cefepime was 60.4 (±30.4)% of the administered dose, and the average renal clearance was 2 (±1.1) mL/min/kg. There were no significant effects of age or gender (25 male vs 17 female) on total body clearance or volume of distribution, corrected for body weight. No accumulation was seen when cefepime was given at 50 mg per kg every 12 hours (n=13), while Cmax, AUC, and t½ were increased about 15% at steady state after 50 mg per kg every 8 hours. The exposure to cefepime following a 50 mg per kg intravenous dose in a pediatric patient is comparable to that in an adult treated with a 2 g intravenous dose. The absolute bioavailability of cefepime after an intramuscular dose of 50 mg per kg was 82.3 (±15)% in eight patients.
Microbiology
Cefepime is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Cefepime has a broad spectrum of in vitro activity that encompasses a wide range of Gram-positive and Gram-negative bacteria. Cefepime has a low affinity for chromosomally-encoded beta-lactamases. Cefepime is highly resistant to hydrolysis by most beta-lactamases and exhibits rapid penetration into Gram-negative bacterial cells. Within bacterial cells, the molecular targets of cefepime are the penicillin binding proteins (PBP).
Cefepime has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobic Gram-Negative Microorganisms:
Enterobacter
Escherichia coli
Klebsiella pneumoniae
Proteus mirabilis
Pseudomonas aeruginosa
Aerobic Gram-Positive Microorganisms:
Staphylococcus aureus
(methicillin-susceptible isolates only)
Streptococcus pneumoniae
Streptococcus pyogenes
(Lancefield’s Group A streptococci)
Viridans group streptococci
The following in vitro data are available, but their clinical significance is unknown . Cefepime has been shown to have in vitro activity against most isolates of the following microorganisms; however, the safety and effectiveness of cefepime in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled trials.
Aerobic Gram-Positive Microorganisms:
Staphylococcus epidermidis
(methicillin-susceptible isolates only)
Staphylococcus saprophyticus
Streptococcus agalactiae
(Lancefield’s Group B streptococci)
NOTE: Most isolates of enterococci, e.g., Enterococcus faecalis , and methicillin-resistant staphylococci are resistant to cefepime.
Aerobic Gram-Negative Microorganisms:
Acinetobacter calcoaceticus
subsp.
lwoffii
Citrobacter diversus
Citrobacter freundii
Enterobacter agglomerans
Haemophilus influenzae
(including beta-lactamase producing isolates)
Hafnia alvei
Klebsiella oxytoca
Moraxella catarrhalis
(including beta-lactamase producing isolates)
Morganella morganii
Proteus vulgaris
Providencia rettgeri
Providencia stuartii
Serratia marcescens
NOTE: Cefepime is inactive against many isolates of Stenotrophomonas (formerly Xanthomonas maltophilia and Pseudomonas maltophilia ).
Anaerobic Microorganisms:
NOTE: Cefepime is inactive against most isolates of Clostridium difficile .
Susceptibility Tests
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method1 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of cefepime powder. The MIC values should be interpreted according to the following criteria:
| Microorganism |
MIC (mcg/mL) |
||
|
Susceptible (S) |
Intermediate (I) |
Resistant (R) |
|
|
Microorganisms other than |
≤8 |
16 |
≥32 |
|
Haemophilus spp.* |
≤2 |
—* |
—* |
|
S. pneumoniae * |
≤0.5 |
1 |
≥2 |
|
*NOTE: Isolates from these species should be tested for susceptibility using specialized dilution testing methods.1 Also, isolates of Haemophilus spp. with MICs greater than 2 mcg/mL should be considered equivocal and should be further evaluated. |
|||
A report of “Susceptible” indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of “Intermediate” indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Laboratory control microorganisms are specific strains of microbiological assay organisms with intrinsic biological properties relating to resistance mechanisms and their genetic expression within bacteria; the specific strains are not clinically significant in their current microbiological status. Standard cefepime powder should provide the following MIC values ( Table 5 ) when tested against the designated quality control strains:
|
Microorganism |
ATCC |
MIC (mcg/mL) |
|
Escherichia coli |
25922 |
0.016 to 0.12 |
|
Staphylococcus aureus |
29213 |
1 to 4 |
|
Pseudomonas aeruginosa |
27853 |
1 to 4 |
|
Haemophilus influenzae |
49247 |
0.5 to 2 |
|
Streptococcus pneumoniae |
49619 |
0.06 to 0.25 |
Diffusion Techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 30 mcg of cefepime to test the susceptibility of microorganisms to cefepime. Interpretation is identical to that stated above for results using dilution techniques.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30 mcg cefepime disk should be interpreted according to the following criteria:
|
Microorganism |
Zone Diameter (mm) |
||
|
Susceptible (S) |
Intermediate (I) |
Resistant (R) |
|
|
Microorganisms other than |
≥18 |
15 to 17 |
≤14 |
|
Haemophilus spp.* |
≥26 |
—* |
—* |
|
*NOTE: Isolates from these species should be tested for susceptibility using specialized diffusion testing methods2. Isolates of Haemophilus spp. with zones smaller than 26 mm should be considered equivocal and should be further evaluated. Isolates of S.pneumoniae should be tested against a 1 mcg oxacillin disk; isolates with oxacillin zone sizes larger than or equal to 20 mm may be considered susceptible to cefepime. |
|||
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Laboratory control microorganisms are specific strains of microbiological assay organisms with intrinsic biological properties relating to resistance mechanisms and their genetic expression within bacteria; the specific strains are not clinically significant in their current microbiological status. For the diffusion technique, the 30 mcg cefepime disk should provide the following zone diameters in these laboratory test quality control strains ( Table 7 ):
|
Microorganism |
ATCC |
Zone Size Range (mm) |
|
Escherichia coli |
25922 |
29 to 35 |
|
Staphylococcus aureus |
25923 |
23 to 29 |
|
Pseudomonas aeruginosa |
27853 |
24 to 30 |
|
Haemophilus influenzae |
49247 |
25 to 31 |
CEFEPIME INDICATIONS AND USAGE
Cefepime for injection is indicated in the treatment of the following infections caused by susceptible strains of the designated microorganisms (see also PRECAUTIONS: Pediatric Use and DOSAGE AND ADMINISTRATION ):
Pneumonia (moderate to severe) caused by Streptococcus pneumoniae , including cases associated with concurrent bacteremia, Pseudomonas aeruginosa , Klebsiella pneumoniae , or Enterobacter species.
Empiric Therapy for Febrile Neutropenic Patients. Cefepime as monotherapy is indicated for empiric treatment of febrile neutropenic patients. In patients at high risk for severe infection (including patients with a history of recent bone marrow transplantation, with hypotension at presentation, with an underlying hematologic malignancy, or with severe or prolonged neutropenia), antimicrobial monotherapy may not be appropriate. Insufficient data exist to support the efficacy of cefepime monotherapy in such patients. (See CLINICAL STUDIES .)
Uncomplicated and Complicated Urinary Tract Infections (including pyelonephritis) caused by Escherichia coli or Klebsiella pneumoniae , when the infection is severe, or caused by Escherichia coli , Klebsiella pneumoniae , or Proteus mirabilis , when the infection is mild to moderate, including cases associated with concurrent bacteremia with these microorganisms.
Uncomplicated Skin and Skin Structure Infections caused by Staphylococcus aureus (methicillin-susceptible strains only) or Streptococcus pyogenes .
Complicated Intra-abdominal Infections (used in combination with metronidazole) caused by Escherichia coli , viridans group streptococci, Pseudomonas aeruginosa , Klebsiella pneumoniae , Enterobacter species, or Bacteroides fragilis . (See CLINICAL STUDIES .)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefepime for injection and other antibacterial drugs, cefepime for injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CLINICAL STUDIES
Febrile Neutropenic Patients
The safety and efficacy of empiric cefepime monotherapy of febrile neutropenic patients have been assessed in two multicenter, randomized trials comparing cefepime monotherapy (at a dose of 2 g intravenously every 8 hours) to ceftazidime monotherapy (at a dose of 2 g intravenously every 8 hours). These studies comprised 317 evaluable patients. Table 8 describes the characteristics of the evaluable patient population.
|
Total |
Cefepime |
Ceftazidime |
|
164 |
153 |
|
|
Median age (yr) |
56 (range, 18 to 82) |
55 (range, 16 to 84) |
|
Male |
86 (52%) |
85 (56%) |
|
Female |
78 (48%) |
68 (44%) |
|
Leukemia |
65 (40%) |
52 (34%) |
|
Other hematologic malignancies |
43 (26%) |
36 (24%) |
|
Solid tumor |
54 (33%) |
56 (37%) |
|
Median ANC nadir (cells/microliter) |
20 (range, 0 to 500) |
20 (range, 0 to 500) |
|
Median duration of neutropenia (days) |
6 (range, 0 to 39) |
6 (range, 0 to 32) |
|
Indwelling venous catheter |
97 (59%) |
86 (56%) |
|
Prophylactic antibiotics |
62 (38%) |
64 (42%) |
|
Bone marrow graft |
9 (5%) |
7 (5%) |
|
SBP less than 90 mm Hg at entry |
7 (4%) |
2 (1%) |
|
ANC = absolute neutrophil count; SBP = systolic blood pressure |
||
Table 9 describes the clinical response rates observed. For all outcome measures, cefepime was therapeutically equivalent to ceftazidime.
|
Outcome Measures |
% Response |
|
|
Cefepime |
Ceftazidime |
|
|
Primary episode resolved with no treatment modification, |
51 |
55 |
|
Primary episode resolved with no treatment modification, |
34 |
39 |
|
Survival, any treatment modification allowed |
93 |
97 |
|
Primary episode resolved with no treatment modification and |
62 |
67 |
|
Primary episode resolved with no treatment modification and |
46 |
51 |
Insufficient data exist to support the efficacy of cefepime monotherapy in patients at high risk for severe infection (including patients with a history of recent bone marrow transplantation, with hypotension at presentation, with an underlying hematologic malignancy, or with severe or prolonged neutropenia). No data are available in patients with septic shock.
Complicated Intra-abdominal Infections
Patients hospitalized with complicated intra-abdominal infections participated in a randomized, double-blind, multicenter trial comparing the combination of cefepime (2 g every 12 hours) plus intravenous metronidazole (500 mg every 6 hours) versus imipenem/cilastatin (500 mg every 6 hours) for a maximum duration of 14 days of therapy. The study was designed to demonstrate equivalence of the two therapies. The primary analyses were conducted on the protocol-valid population, which consisted of those with a surgically confirmed complicated infection, at least one pathogen isolated pretreatment, at least 5 days of treatment, and a 4 to 6 week follow-up assessment for cured patients. Subjects in the imipenem/cilastatin arm had higher APACHE II scores at baseline. The treatment groups were otherwise generally comparable with regard to their pretreatment characteristics. The overall clinical cure rate among the protocol-valid patients was 81% (51 cured/63 evaluable patients) in the cefepime plus metronidazole group and 66% (62/94) in the imipenem/cilastatin group. The observed differences in efficacy may have been due to a greater proportion of patients with high APACHE II scores in the imipenem/cilastatin group.
CEFEPIME CONTRAINDICATIONS
Cefepime for injection is contraindicated in patients who have shown immediate hypersensitivity reactions to cefepime or the cephalosporin class of antibiotics, penicillins or other beta-lactam antibiotics.
WARNINGS
Hypersensitivity Reactions to Cefepime, Cephalosporins, Penicillins, or Other Drugs
Before therapy with Cefepime for Injection is instituted, careful inquiry should be made to determine whether the patient has had previous immediate hypersensitivity reactions to cefepime, cephalosporins, penicillins, or other drugs. Exercise caution if this product is to be given to penicillin-sensitive patients because cross-hypersensitivity among beta-lactam antibiotics has been clearly documented and may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction to Cefepime for Injection occurs, discontinue the drug.
Use in Patients with Renal Impairment
In patients with creatinine clearance less than or equal to 60 mL/min, adjust the dose of cefepime (cefepime hydrochloride) to compensate for the slower rate of renal elimination [see DOSAGE AND ADMINISTRATION ]. Because high and prolonged serum cefepime concentrations can occur from usual dosages in patients with renal impairment, the cefepime dosage should be reduced when it is administered to such patients. Continued dosage should be determined by degree of renal impairment, severity of infection, and susceptibility of the causative organisms.
Neurotoxicity
During postmarketing surveillance, serious adverse reactions have been reported including life-threatening or fatal occurrences of the following: encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, seizures, and nonconvulsive status epilepticus (see ADVERSE REACTIONS: Postmarketing Experience ). Most cases occurred in patients with renal impairment who did not receive appropriate dosage adjustment. However, some cases of neurotoxicity occurred in patients receiving a dosage adjustment appropriate for their degree of renal impairment. In the majority of cases, symptoms of neurotoxicity were reversible and resolved after discontinuation of cefepime and/or after hemodialysis. If neurotoxicity associated with cefepime therapy occurs, consider discontinuing cefepime or making appropriate dosage adjustments in patients with renal impairment.
Clostridium difficile Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefepime, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile , and surgical evaluation should be instituted as clinically indicated.
PRECAUTIONS
General
Prescribing cefepime for injection in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
As with other antimicrobials, prolonged use of cefepime may result in overgrowth of nonsusceptible microorganisms. Repeated evaluation of the patient’s condition is essential. Should superinfection occur during therapy, appropriate measures should be taken.
Many cephalosporins, including cefepime, have been associated with a fall in prothrombin activity. Those at risk include patients with renal or hepatic impairment, or poor nutritional state, as well as patients receiving a protracted course of antimicrobial therapy. Prothrombin time should be monitored in patients at risk, and exogenous vitamin K administered as indicated.
Positive direct Coombs’ tests have been reported during treatment with cefepime. In hematologic studies or in transfusion cross-matching procedures when antiglobulin tests are performed on the minor side or in Coombs’ testing of newborns whose mothers have received cephalosporin antibiotics before parturition, it should be recognized that a positive Coombs’ test may be due to the drug.
Cefepime for injection should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Arginine has been shown to alter glucose metabolism and elevate serum potassium transiently when administered at 33 times the amount provided by the maximum recommended human dose of cefepime. The effect of lower doses is not presently known.
Information for Patients
Before therapy with Cefepime for Injection is instituted, careful inquiry should be made to determine whether the patient has had previous immediate hypersensitivity reactions to cefepime, cephalosporins, penicillins, or other drugs. Exercise caution if this product is to be given to penicillin-sensitive patients because cross-hypersensitivity among beta-lactam antibiotics has been clearly documented and may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction to Cefepime for Injection occurs, discontinue the drug. Serious acute hypersensitivity reactions may require treatment with epinephrine and other emergency measures including oxygen, corticosteroids, intravenous fluids, intravenous antihistamines, pressor amines, and airway management, as clinically indicated.
Patients should be counseled that antibacterial drugs including cefepime for injection should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefepime for injection is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefepime for injection or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics, which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Patients should be advised of neurological adverse events that could occur with cefepime for injection use. Patients should be instructed to inform their healthcare provider at once of any neurological signs and symptoms, including encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, seizures, and nonconvulsive status epilepticus for immediate treatment, dosage adjustment, or discontinuation of cefepime for injection.
Drug Interactions
Renal function should be monitored carefully if high doses of aminoglycosides are to be administered with cefepime because of the increased potential of nephrotoxicity and ototoxicity of aminoglycoside antibiotics. Nephrotoxicity has been reported following concomitant administration of other cephalosporins with potent diuretics such as furosemide.
Drug/Laboratory Test Interactions
The administration of cefepime may result in a false-positive reaction for glucose in the urine when using ClinitestTM tablets. It is recommended that glucose tests based on enzymatic glucose oxidase reactions (such as ClinistixTM) be used.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal carcinogenicity studies have been conducted with cefepime. In chromosomal aberration studies, cefepime was positive for clastogenicity in primary human lymphocytes, but negative in Chinese hamster ovary cells. In other in vitro assays (bacterial and mammalian cell mutation, DNA repair in primary rat hepatocytes, and sister chromatid exchange in human lymphocytes), cefepime was negative for genotoxic effects. Moreover, in vivo assessments of cefepime in mice (2 chromosomal aberration and 2 micronucleus studies) were negative for clastogenicity. No untoward effects on fertility were observed in rats when cefepime was administered subcutaneously at doses up to 1000 mg/kg/day (1.6 times the recommended maximum human dose calculated on a mg/m2 basis).
Pregnancy
Teratogenic Effects: Pregnancy Category B
Cefepime was not teratogenic or embryocidal when administered during the period of organogenesis to rats at doses up to 1000 mg/kg/day (1.6 times the recommended maximum human dose calculated on a mg/m2 basis) or to mice at doses up to 1200 mg/kg (approximately equal to the recommended maximum human dose calculated on a mg/m2 basis) or to rabbits at a dose level of 100 mg/kg (0.3 times the recommended maximum human dose calculated on a mg/m2 basis).
There are, however, no adequate and well-controlled studies of cefepime use in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Cefepime is excreted in human breast milk in very low concentrations (0.5 mcg/mL). Caution should be exercised when cefepime is administered to a nursing woman.
Labor and Delivery
Cefepime has not been studied for use during labor and delivery. Treatment should only be given if clearly indicated.
Pediatric Use
The safety and effectiveness of cefepime in the treatment of uncomplicated and complicated urinary tract infections (including pyelonephritis), uncomplicated skin and skin structure infections, pneumonia, and as empiric therapy for febrile neutropenic patients have been established in the age groups 2 months up to 16 years. Use of cefepime in these age groups is supported by evidence from adequate and well-controlled studies of cefepime in adults with additional pharmacokinetic and safety data from pediatric trials (see CLINICAL PHARMACOLOGY ).
Safety and effectiveness in pediatric patients below the age of 2 months have not been established. There are insufficient clinical data to support the use of cefepime for injection in pediatric patients under 2 months of age or for the treatment of serious infections in the pediatric population where the suspected or proven pathogen is Haemophilus influenzae type b.
IN THOSE PATIENTS IN WHOM MENINGEAL SEEDING FROM A DISTANT INFECTION SITE OR IN WHOM MENINGITIS IS SUSPECTED OR DOCUMENTED, AN ALTERNATE AGENT WITH DEMONSTRATED CLINICAL EFFICACY IN THIS SETTING SHOULD BE USED.
Geriatric Use
Of the more than 6400 adults treated with cefepime for injection in clinical studies, 35% were 65 years or older while 16% were 75 years or older. When geriatric patients received the usual recommended adult dose, clinical efficacy and safety were comparable to clinical efficacy and safety in nongeriatric adult patients.
Serious adverse events have occurred in geriatric patients with renal insufficiency given unadjusted doses of cefepime, including life-threatening or fatal occurrences of the following: encephalopathy, myoclonus, and seizures. (See WARNINGS and ADVERSE REACTIONS. )
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and renal function should be monitored. (See CLINICAL PHARMACOLOGY: Specific Populations, WARNINGS, and DOSAGE AND ADMINISTRATION. )
CEFEPIME ADVERSE REACTIONS
Clinical Trials
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials using multiple doses of cefepime, 4137 patients were treated with the recommended dosages of cefepime (500 mg to 2 g intravenous every 12 hours). There were no deaths or permanent disabilities thought related to drug toxicity. Sixty-four (1.5%) patients discontinued medication due to adverse events thought by the investigators to be possibly, probably, or almost certainly related to drug toxicity. Thirty-three (51%) of these 64 patients who discontinued therapy did so because of rash. The percentage of cefepime-treated patients who discontinued study drug because of drug-related adverse events was very similar at daily doses of 500 mg, 1 g, and 2 g every 12 hours (0.8%, 1.1%, and 2%, respectively). However, the incidence of discontinuation due to rash increased with the higher recommended doses.
The following adverse events were thought to be probably related to cefepime during evaluation of the drug in clinical trials conducted in North America (n=3125 cefepime-treated patients).
|
Cefepime Multiple-Dose Dosing Regimens |
|
| Clinical Trials-North America | |
|
INCIDENCE EQUAL TO OR GREATER THAN 1% |
Local reactions (3%), including phlebitis (1.3%), pain and/or inflammation (0.6%)*; rash (1.1%) |
|
INCIDENCE LESS THAN 1% BUT GREATER THAN 0.1% |
Colitis (including pseudomembranous colitis), diarrhea, erythema, fever, headache, nausea, oral moniliasis, pruritus, urticaria, vaginitis, vomiting, anemia |
|
*Local reactions, irrespective of relationship to cefepime in those patients who received intravenous infusion (n=3048). |
|
At the higher dose of 2 g every 8 hours, the incidence of probably-related adverse events was higher among the 795 patients who received this dose of cefepime. They consisted of rash (4%), diarrhea (3%), nausea (2%), vomiting (1%), pruritus (1%), fever (1%), and headache (1%).
The following adverse laboratory changes, irrespective of relationship to therapy with cefepime, were seen during clinical trials conducted in North America.
| Cefepime Multiple-Dose Dosing Regimens | |
| Clinical Trials-North America | |
|
INCIDENCE EQUAL TO OR GREATER THAN 1% |
Positive Coombs’ test (without hemolysis) (16.2%); decreased phosphorus (2.8%); increased ALT/SGPT (2.8%), AST/SGOT (2.4%), eosinophils (1.7%); abnormal PTT (1.6%), PT (1.4%) |
|
INCIDENCE LESS THAN 1% BUT GREATER THAN 0.1% |
Increased alkaline phosphatase, BUN, calcium, creatinine, phosphorus, potassium, total bilirubin; decreased calcium*, hematocrit, neutrophils, platelets, WBC |
|
*Hypocalcemia was more common among elderly patients. Clinical consequences from changes in either calcium or phosphorus were not reported. |
|
A similar safety profile was seen in clinical trials of pediatric patients (see PRECAUTIONS: Pediatric Use ).
Postmarketing Experience
In addition to the events reported during North American clinical trials with cefepime, the following adverse experiences have been reported during worldwide postmarketing experience.
Encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, seizures, and nonconvulsive status epilepticus have been reported. Although most cases occurred in patients with renal impairment who received doses of cefepime that exceeded the recommended dosage schedules, some cases of neurotoxicity occurred in patients receiving an appropriate dosage adjustment for their degree of renal impairment. If neurotoxicity associated with cefepime therapy occurs, consider discontinuing cefepime or making appropriate dosage adjustments in patients with renal impairment. (See WARNINGS ).
As with other cephalosporins, anaphylaxis including anaphylactic shock, transient leukopenia, neutropenia, agranulocytosis and thrombocytopenia have been reported.
Cephalosporin-Class Adverse Reactions
In addition to the adverse reactions listed above that have been observed in patients treated with cefepime, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics:
Stevens-Johnson syndrome, erythema multiforme, toxic epidermal necrolysis, renal dysfunction, toxic nephropathy, aplastic anemia, hemolytic anemia, hemorrhage, hepatic dysfunction including cholestasis, and pancytopenia.
OVERDOSAGE
Patients who receive an overdose should be carefully observed and given supportive treatment. In the presence of renal insufficiency, hemodialysis, not peritoneal dialysis, is recommended to aid in the removal of cefepime from the body. Accidental overdosing has occurred when large doses were given to patients with impaired renal function. Symptoms of overdose include encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, seizures, neuromuscular excitability and nonconvulsive status epilepticus. (See WARNINGS, ADVERSE REACTIONS , and DOSAGE AND ADMINISTRATION .)
CEFEPIME DOSAGE AND ADMINISTRATION
The recommended adult and pediatric dosages and routes of administration are outlined in the following table. Cefepime for injection should be administered intravenously over approximately 30 minutes.
|
Site and Type of Infection |
Dose |
Frequency |
Duration
|
| Adults | |||
|
Moderate to Severe Pneumonia due to S. pneumoniae *, P. aeruginosa , K. pneumoniae , or Enterobacter species |
1 to 2 g IV |
Every 12 hours |
10 |
|
Empiric therapy for febrile neutropenic patients (See INDICATIONS AND USAGE and CLINICAL STUDIES .) |
2 g IV |
Every 8 hours |
7** |
|
Mild to Moderate Uncomplicated or Complicated Urinary Tract Infections, including pyelonephritis, due to E. coli , K. pneumoniae , or P. mirabilis * |
0.5 to 1 g |
Every 12 hours |
7 to 10 |
|
Severe Uncomplicated or Complicated Urinary Tract Infections, including pyelonephritis, due to E. coli or K.pneumoniae * |
2 g IV |
Every 12 hours |
10 |
|
Moderate to Severe Uncomplicated Skin and Skin Structure Infections due to S. aureus or S. pyogenes |
2 g IV |
Every 12 hours |
10 |
|
Complicated Intra-abdominal Infections (used in combination with metronidazole) caused by E. coli , viridans group streptococci, P. aeruginosa , K.pneumoniae , Enterobacter species, or B. fragilis . (See CLINICAL STUDIES .) |
2 g IV |
Every 12 hours |
7 to 10 |
|
Pediatric Patients (2 months up to 16 years) The maximum dose for pediatric patients should not exceed the recommended adult dose. The usual recommended dosage in pediatric patients up to 40 kg in weight for uncomplicated and complicated urinary tract infections (including pyelonephritis), uncomplicated skin and skin structure infections, and pneumonia is 50 mg per kg per dose, administered every 12 hours (50 mg per kg per dose, every 8 hours for febrile neutropenic patients), for durations as given above. |
|||
|
*including cases associated with concurrent bacteremia **or until resolution of neutropenia. In patients whose fever resolves but who remain neutropenic for more than 7 days, the need for continued antimicrobial therapy should be re-evaluated frequently. ***Intramuscular route of administration is indicated only for mild to moderate, uncomplicated or complicated UTIs due to E. coli when the intramuscular route is considered to be a more appropriate route of drug administration. |
|||
Patients with Hepatic Impairment
No adjustment is necessary for patients with hepatic impairment.
Patients with Renal Impairment
In patients with creatinine clearance less than or equal to 60 mL/min, the dose of cefepime should be adjusted to compensate for the slower rate of renal elimination. The recommended initial dose of cefepime should be the same as in patients with normal renal function except in patients undergoing hemodialysis. The recommended doses of cefepime in patients with renal impairment are presented in Table 13 .
When only serum creatinine is available, the following formula (Cockcroft and Gault equation)3 may be used to estimate creatinine clearance. The serum creatinine should represent a steady state of renal function:
| Males: Creatinine Clearance (mL/min) | =
Weight (kg) x (140–age)
72 x serum creatinine (mg/dL) |
| Females: 0.85 x above value | |
|
Creatinine |
Recommended Maintenance Schedule | ||||
|
Greater than 60 |
500 mg |
1 g |
2 g |
2 g |
|
|
30 to 60 |
500 mg |
1 g |
2 g |
2 g |
|
|
11 to 29 |
500 mg |
500 mg |
1 g |
2 g |
|
|
Less than 11 |
250 mg |
250 mg |
500 mg |
1 g |
|
|
CAPD |
500 mg |
1 g |
2 g |
2 g |
|
|
Hemodialysis* |
1 g on day 1, then 500 mg every 24 hours thereafter |
1 g |
|||
|
*On hemodialysis days, cefepime should be administered following hemodialysis. Whenever possible, cefepime should be administered at the same time each day. |
|||||
In patients undergoing continuous ambulatory peritoneal dialysis, cefepime for injection may be administered at normally recommended doses at a dosage interval of every 48 hours (see Table 13 ).
In patients undergoing hemodialysis, approximately 68% of the total amount of cefepime present in the body at the start of dialysis will be removed during a 3 hour dialysis period. The dosage of cefepime for injection for hemodialysis patients is 1 g on Day 1 followed by 500 mg every 24 hours for the treatment of all infections except febrile neutropenia, which is 1 g every 24 hours. Cefepime for injection should be administered at the same time each day and following the completion of hemodialysis on hemodialysis days (see Table 13 ).
Data in pediatric patients with impaired renal function are not available; however, since cefepime pharmacokinetics are similar in adults and pediatric patients (see CLINICAL PHARMACOLOGY ), changes in the dosing regimen proportional to those in adults (see Tables 12 and 13 ) are recommended for pediatric patients.
Administration
For Intravenous Infusion
Dilute with a suitable parenteral vehicle prior to intravenous infusion. Constitute the 500 mg, 1 g, or 2 g vial, and add an appropriate quantity of the resulting solution to an intravenous container with one of the compatible intravenous fluids listed in the Compatibility and Stability subsection. THE RESULTING SOLUTION SHOULD BE ADMINISTERED OVER APPROXIMATELY 30 MINUTES.
Intermittent intravenous infusion with a Y-type administration set can be accomplished with compatible solutions. However, during infusion of a solution containing cefepime, it is desirable to discontinue the other solution.
Intramuscular Administration
For intramuscular administration, cefepime hydrochloride should be constituted with one of the following diluents: Sterile Water for Injection, 0.9% Sodium Chloride, 5% Dextrose Injection, 0.5% or 1% Lidocaine Hydrochloride, or Sterile Bacteriostatic Water for Injection with Parabens or Benzyl Alcohol (refer to Table 14 ).
Preparation of cefepime for injection solutions is summarized in Table 14 .
|
Single-Dose Vials for Intravenous/Intramuscular Administration |
Amount of Diluent |
Approximate |
Approximate |
|
cefepime vial content |
|
|
|
|
500 mg (IV) |
5 |
5.6 |
100 |
|
500 mg (IM) |
1.3 |
1.8 |
280 |
|
1 g (IV) |
10 |
11.3 |
100 |
|
1 g (IM) |
2.4 |
3.6 |
280 |
|
2 g (IV) |
10 |
12.5 |
160 |
Compatibility and Stability
Intravenous
Cefepime for injection is compatible at concentrations between 1 mg per mL and 40 mg per mL with the following intravenous infusion fluids: 0.9% Sodium Chloride Injection, 5% and 10% Dextrose Injection, M/6 Sodium Lactate Injection, 5% Dextrose and 0.9% Sodium Chloride Injection, Lactated Ringers and 5% Dextrose Injection, NormosolTM-R, and NormosolTM-M in 5% Dextrose Injection. These solutions may be stored up to 24 hours at controlled room temperature 20° to 25° C (68° to 77° F) or 7 days in a refrigerator 2° to 8° C (36° to 46° F).
Cefepime for injection admixture compatibility information is summarized in Table 15.
|
Cefepime |
Admixture and Concentration |
IV Infusion Solutions |
Stability Time for | |
|
RT/L |
Refrigeration (2°to 8° C) |
|||
|
40 mg/mL |
Amikacin |
NS or D5W |
24 hours |
7 days |
|
40 mg/mL |
Ampicillin |
D5W |
8 hours |
8 hours |
|
40 mg/mL |
Ampicillin |
D5W |
2 hours |
8 hours |
|
40 mg/mL |
Ampicillin |
NS |
24 hours |
48 hours |
|
40 mg/mL |
Ampicillin |
NS |
8 hours |
48 hours |
|
4 mg/mL |
Ampicillin |
NS |
8 hours |
8 hours |
|
4 to 40 mg/mL |
Clindamycin |
NS or D5W |
24 hours |
7 days |
|
4 mg/mL |
Heparin |
NS or D5W |
24 hours |
7 days |
|
4 mg/mL |
Potassium Chloride |
NS or D5W |
24 hours |
7 days |
|
4 mg/mL |
Theophylline |
D5W |
24 hours |
7 days |
|
1 to 4 mg/mL |
na |
AminosynTM II |
8 hours |
3 days |
|
0.125 to 0.25 mg/mL |
na |
InpersolTM |
24 hours |
7 days |
|
NS = 0.9% Sodium Chloride Injection D5W = 5% Dextrose Injection na = not applicable RT/L = Ambient room temperature and light |
||||
Solutions of cefepime for injection, like those of most beta-lactam antibiotics, should not be added to solutions of ampicillin at a concentration greater than 40 mg per mL, and should not be added to metronidazole, vancomycin, gentamicin, tobramycin, netilmicin sulfate, or aminophylline because of potential interaction. However, if concurrent therapy with cefepime is indicated, each of these antibiotics can be administered separately.
Intramuscular
Cefepime for injection constituted as directed is stable for 24 hours at controlled room temperature 20° to 25° C (68° to 77° F) or for 7 days in a refrigerator 2° to 8° C (36° to 46° F) with the following diluents: Sterile Water for Injection, 0.9% Sodium Chloride Injection, 5% Dextrose Injection, Sterile Bacteriostatic Water for Injection with Parabens or Benzyl Alcohol, or 0.5% or 1% Lidocaine Hydrochloride.
NOTE: PARENTERAL DRUGS SHOULD BE INSPECTED VISUALLY FOR PARTICULATE MATTER BEFORE ADMINISTRATION. IF PARTICULATE MATTER IS EVIDENT IN RECONSTITUTED FLUIDS, THE DRUG SOLUTION SHOULD BE DISCARDED.
As with other cephalosporins, the color of cefepime powder, as well as its solutions, tend to darken depending on storage conditions; however, when stored as recommended, the product potency is not adversely affected.
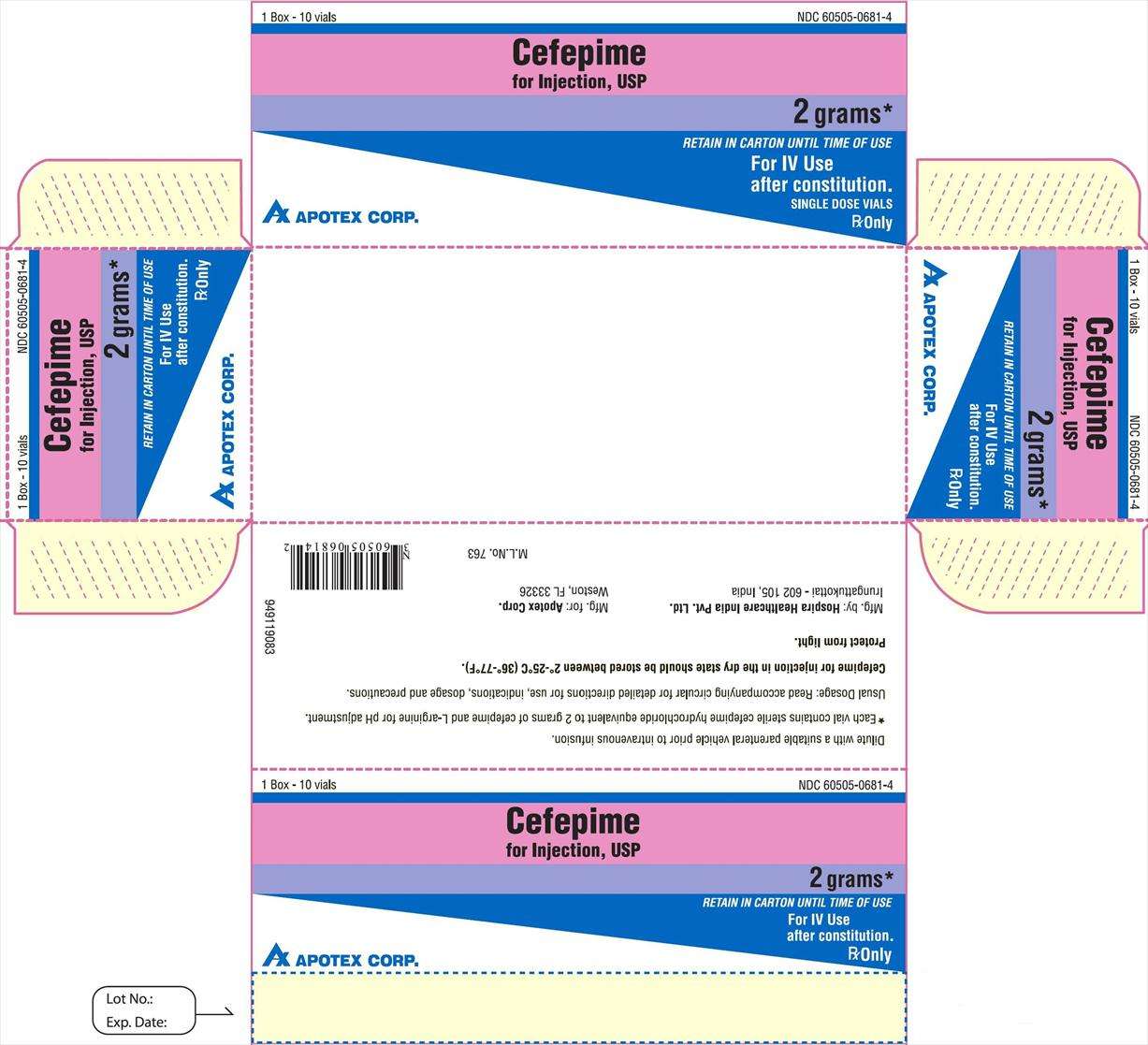
HOW SUPPLIED
Each vial contains sterile cefepime hydrochloride equivalent to 500 mg, 1 g or 2 g of cefepime and L- arginine for pH adjustment.
Cefepime for injection, USP is supplied as a sterile powder in glass vials as follows:
Vials containing 500 mg equivalent of cefepime. Box of 1 (NDC 60505–0678–0) and box of 10 (NDC 60505–0678–4).
Vials containing 1 g equivalent of cefepime. Box of 1 (NDC 60505–0834–0) and box of 10 (NDC 60505–0834–4).
Vials containing 2 g equivalent of cefepime. Box of 1 (NDC 60505–0681–0) and box of 10 (NDC 60505–0681–4).
Storage
CEFEPIME FOR INJECTION IN THE DRY STATE SHOULD BE STORED BETWEEN 2° to 25° C (36° to 77° F) AND PROTECTED FROM LIGHT.
REFERENCES
(1) National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically -Third Edition. Approved Standard NCCLS Document M7-A3, Vol. 13, No. 25, NCCLS, Villanova, PA, December 1993.
(2) National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests - Fifth Edition. Approved Standard NCCLS Document M2-A5, Vol. 13, No. 24, NCCLS, Villanova, PA, December 1993.
(3) Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron . 1976; 16:31-41.
Mfg. by: Mfg. for:
Hospira Healthcare India Pvt. Ltd. Apotex Corp.
Irungattukottai - 602 105, India Weston, FL 33326
DATE OF REVISION: April 2013
948026270
9491xxxxx

949119082

949119083
CefepimeCEFEPIME INJECTION, POWDER, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CefepimeCEFEPIME INJECTION, POWDER, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CefepimeCEFEPIME INJECTION, POWDER, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||