Cefdinir
Dispensing Solutions, Inc.
PSS World Medical, Inc.
Cefdinir for Oral Suspension USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- CEFDINIR DESCRIPTION
- CEFDINIR INDICATIONS AND USAGE
- CEFDINIR CONTRAINDICATIONS
- WARNINGS
- OVERDOSAGE
- CEFDINIR DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
125 mg/5 mL and 250 mg/5 mL
Rx only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefdinir for oral suspension and other antibacterial drugs, cefdinir for oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
CEFDINIR DESCRIPTION
Cefdinir for oral suspension contains the active ingredient cefdinir, an extended-spectrum, semisynthetic cephalosporin, for oral administration. Chemically, cefdinir is [6R-[6α,7β(Z)]]-7-[[(2-amino-4- thiazolyl)(hydroxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. Cefdinir is a white to slightly brownish-yellow solid. It is slightly soluble in dilute hydrochloric acid and sparingly soluble in 0.1 M pH 7.0 phosphate buffer. The molecular formula is C14H13N5O5S2 and the molecular weight is 395.42. Cefdinir has the structural formula shown below:
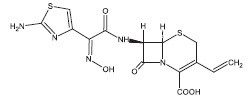
Cefdinir
Cefdinir for oral suspension, after reconstitution, contains 125 mg cefdinir per 5 mL or 250 mg cefdinir per 5 mL and the following inactive ingredients: anhydrous citric acid; colloidal silicon dioxide; guar gum; anhydrous sodium citrate; sodium benzoate; strawberry flavour; sucrose; and xanthan gum.
CEFDINIR INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefdinir for oral suspension and other antibacterial drugs, cefdinir for oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Cefdinir for oral suspension is indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
Adults and Adolescents:
Enter section text here
Pediatric Patients:
Enter section text here
CEFDINIR CONTRAINDICATIONS
Cefdinir is contraindicated in patients with known allergy to the cephalosporin class of antibiotics.
WARNINGS
BEFORE THERAPY WITH CEFDINIR IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFDINIR, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF CEFDINIR IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-HYPERSENSITIVITY AMONG β-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFDINIR OCCURS, THE DRUG SHOULD BE DISCONTINUED. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefdinir, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
OVERDOSAGE
Information on cefdinir overdosage in humans is not available. In acute rodent toxicity studies, a single oral 5600 mg/kg dose produced no adverse effects. Toxic signs and symptoms following overdosage with other β-lactam antibiotics have included nausea, vomiting, epigastric distress, diarrhea, and convulsions. Hemodialysis removes cefdinir from the body. This may be useful in the event of a serious toxic reaction from overdosage, particularly if renal function is compromised.
CEFDINIR DOSAGE AND ADMINISTRATION
(see INDICATIONS AND USAGE for Indicated Pathogens)
The recommended dosage and duration of treatment for infections in pediatric patients are described in the following chart; the total daily dose for all infections is 14 mg/kg, up to a maximum dose of 600 mg per day. Once-daily dosing for 10 days is as effective as BID dosing. Once-daily dosing has not been studied in skin infections; therefore, cefdinir for oral suspension should be administered twice daily in this infection. Cefdinir for oral suspension may be administered without regard to meals.
|
Type
of
Infection
|
Dosage
|
Duration
|
| Acute Bacterial Otitis Media |
7 mg/kg q12h or |
5 to 10 days |
|
|
14 mg/kg q24h |
10 days |
| Acute Maxillary Sinusitis |
7 mg/kg q12h or |
10 days |
|
|
14 mg/kg q24h |
10 days |
| Pharyngitis/Tonsilitis |
7 mg/kg q12h or |
5 to 10 days |
|
|
14 mg/kg q24h |
10 days |
| Uncomplicated Skin and Skin Structure Infections |
7 mg/kg q12h |
10 days |
|
Weight
|
125
mg
/
5
mL
|
250
mg
/
5
mL
|
|
|
||
| 9 kg/20 lbs |
2.5 mL q12h or 5 mL q24h |
Use 125 mg/5 mL product |
| 18 kg/40 lbs |
5 mL q12h or 10 mL q24h |
2.5 mL q12h or 5 mL q24h |
| 27 kg/60 lbs |
7.5 mL q12h or 15 mL q24h |
3.75 mL q12h or 7.5 mL q24h |
| 36 kg/80 lbs |
10 mL q12h or 20 mL q24h |
5 mL q12h or 10 mL q24h |
| ≥43 kg a/95 lbs |
12 mL q12h or 24 mL q24h |
6 mL q12h or 12 mL q24h |
Patients With Renal Insufficiency:
For adult patients with creatinine clearance less than 30 mL/min, the dose of cefdinir should be 300 mg given once daily.
Creatinine clearance is difficult to measure in outpatients. However, the following formula may be used to estimate creatinine clearance (CLcr) in adult patients. For estimates to be valid, serum creatinine levels should reflect steady-state levels of renal function.
(weight) (140 – age)
Males: CLcr = ————————————
(72) (serum creatinine)
Females: CLcr = 0.85 x above value
where creatinine clearance is in mL/min, age is in years, weight is in kilograms, and serum creatinine is in mg/dL.(3)
The following formula may be used to estimate creatinine clearance in pediatric patients:
body length or height
CLcr = K x ———————————
serum creatinine
where K = 0.55 for pediatric patients older than 1 year(4) and 0.45 for infants (up to 1 year)(5).
In the above equation, creatinine clearance is in mL/min/1.73 m2, body length or height is in centimeters, and serum creatinine is in mg/dL.
For pediatric patients with a creatinine clearance of less than 30 mL/min/1.73 m2, the dose of cefdinir should be 7 mg/kg (up to 300 mg) given once daily.
Patients on Hemodialysis:
Hemodialysis removes cefdinir from the body. In patients maintained on chronic hemodialysis, the recommended initial dosage regimen is a 300 mg or 7 mg/kg dose every other day. At the conclusion of each hemodialysis session, 300 mg (or 7 mg/kg) should be given. Subsequent doses (300 mg or 7 mg/kg) are then administered every other day.
|
Final
Concentration
|
Final
Volume
(
mL
)
|
Amount
of
Water
|
Directions
|
| 125 mg/5 mL |
60 |
35 mL |
Tap bottle to loosen the powder, then add water in 2 |
|
|
100 |
58 mL |
portions. Shake well after each aliquot. |
| 250 mg/5 mL |
60 |
35 mL |
Tap bottle to loosen the powder, then add water in 2 |
|
|
100 |
58 mL |
portions. Shake well after each aliquot. |
After mixing, the suspension can be stored at 20°-25°C (68°-77°F). The container should be kept tightly closed, and the suspension should be shaken well before each administration. The suspension may be used for 10 days, after which any unused portion must be discarded.
HOW SUPPLIED
Cefdinir for oral suspension USP, is an off-white to creamish powder formulation that, when reconstituted as directed, contains 125 mg cefdinir/5 mL or 250 mg cefdinir/5 mL. The reconstituted suspension has an off-white to creamish color and strawberry flavor. The powder is available as follows:
125 mg/5 mL:
60 mL bottles NDC 68180-722-20
100 mL bottles NDC 68180-722-10
250 mg/5 mL:
60 mL bottles NDC 68180-723-20
100 mL bottles NDC 68180-723-10
Store dry powder and reconstituted suspension at 20°-25°C (68°-77°F); [see USP Controlled Room Temperature].
REFERENCES
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 4th ed. Approved Standard, NCCLS Document M7-A4, Vol 17(2). NCCLS, Villanova, PA, Jan 1997.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests, 6th ed. Approved Standard, NCCLS Document M2-A6, Vol 17(1). NCCLS, Villanova, PA, Jan 1997.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron, 1976;16:31-41.
- Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976;58:259-63.
- Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatrics 1984;104:849-54.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States
Manufactured by:
Lupin Limited
Mandideep 462 046
INDIA
Clinistix® and Clinitest® are registered trademarks of Miles Diagnostics.
Tes-tape® is a registered trademark of Lilly.
Revised December 15, 2009 ID#: 218627
PRINCIPAL DISPLAY PANEL

NDC 68258-3053-01
CefdinirCefdinir POWDER, FOR SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||