Cefadroxil
PD-Rx Pharmaceuticals, Inc.
PD-Rx Pharmaceuticals, Inc.
cefadroxil capsules, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- CEFADROXIL DESCRIPTION
- CLINICAL PHARMACOLOGY
- CEFADROXIL INDICATIONS AND USAGE
- CEFADROXIL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CEFADROXIL ADVERSE REACTIONS
- OVERDOSAGE
- CEFADROXIL DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mg (50 Capsule Bottle)
FULL PRESCRIBING INFORMATION
CEFADROXIL DESCRIPTION
1617352
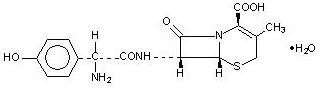
Cefadroxil capsules contain the following inactive ingredients: Lactose monohydrate, magnesium stearate, FD&C Blue No.1, D&C Red No.28, FD&C Red No. 40, titanium dioxide, gelatin, sodium lauryl sulphate, and edible black ink (black iron oxide).
CLINICAL PHARMACOLOGY
Microbiology
In vitroin vitro INDICATIONS AND USAGE
Beta-hemolytic streptococci
Staphylococci,
Streptococcus (Diplococcus) pneumoniae
Escherichia coli
Proteus mirabilis
Klebsiella species
Moraxella (Branhamella) catarrhalis
Note:Enterococcus faecalisStreptococcus faecalisEnterococcus faeciumStreptococcus faeciumEnterobacterMorganella morganiiProteus morganiiP. vulgarisPseudomonasAcinetobacter calcoaceticusMimaHerellea
Susceptibility tests: Diffusion techniques
1
|
Zone diameter (mm)
|
Interpretation
|
| ≥ 18 15–17 ≤ 14 |
(S) Susceptible (I) Intermediate (R) Resistant |
|
Organism
|
Zone Diameter (mm)
|
|
Staphylococcus aureus ATCC 25923 Escherichia coli ATCC 25922 |
29–37 17–22 |
Dilution Techniques
2
Staphylococcus aureusEscherichia coliStreptococcus faecalis
CEFADROXIL INDICATIONS AND USAGE
E. coli, P. mirabilis, Klebsiella
Streptococcus pyogenes
Note:
Note:
CEFADROXIL CONTRAINDICATIONS
WARNINGS
Clostridium difficile C. difficile
C. difficile C. difficile
C. difficileC. difficile
PRECAUTIONS
General
2 DOSAGE AND ADMINISTRATION
Information for Patients
Drug/Laboratory Test Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Labor and Delivery
Nursing Mothers
Pediatric Use
DOSAGE AND ADMINISTRATION
Geriatric Use
DOSAGE AND ADMINISTRATION: Renal Impairment
CEFADROXIL ADVERSE REACTIONS
Gastrointestinal
WARNINGS
Hypersensitivity
Other
DOSAGE AND ADMINISTRATION OVERDOSAGE
OVERDOSAGE
CEFADROXIL DOSAGE AND ADMINISTRATION
Adults
Urinary Tract Infections:
Skin and Skin Structure Infections:
Pharyngitis and Tonsillitis:
Children
Renal Impairment
2
|
Creatinine Clearances
|
Dosage Interval
|
| 0-10 mL/min |
36 hours |
| 10-25 mL/min |
24 hours |
| 25-50 mL/min |
12 hours |
HOW SUPPLIED
Cefadroxil Capsules, USP 500 mg
Store at
REFERENCES
- National Committee for Clinical Laboratory Standards, Approved Standard, Performance Standards for Antimicrobial Disk Susceptibility Test, 4th Edition. Vol. 10 (7): M2-A4, Villanova, PA, April, 1990.
- National Committee for Clinical Laboratory Standards, Approved Standard: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 2nd Edition, Vol. 10(8): M7-A2, Villanova, PA, April, 1990.
GREENSTONE® BRAND
Distributed by:
Greenstone LLC
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mg (50 Capsule Bottle)
GREENSTONE® BRAND
cefadroxil capsules, USP
500 mg
Rx only

CefadroxilCefadroxil CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!