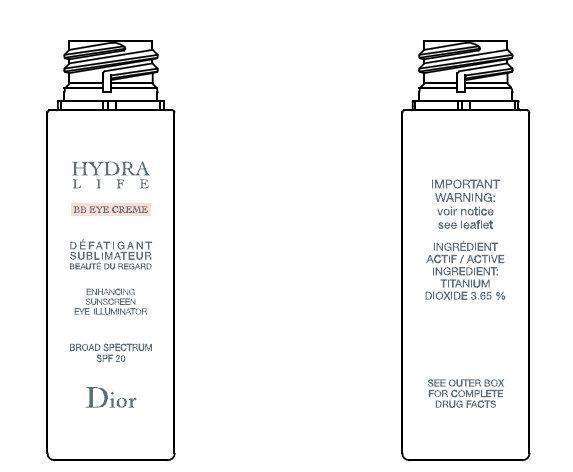
CD HydraLife BB Eye Creme Enhancing Sunscreen Eye Illuminator Luminous Beige Broad Spectrum SPF 20
CD HydraLife BB Eye Creme Enhancing Sunscreen Eye Illuminator Luminous Beige Broad Spectrum SPF 20
FULL PRESCRIBING INFORMATION: CONTENTS*
- CD HydraLife BB Eye Creme Enhancing Sunscreen Eye Illuminator Luminous Beige Broad Spectrum SPF 20
- Active ingredients
- Purpose
- CD HydraLife BB Eye Creme Enhancing Sunscreen Eye Illuminator Luminous Beige Broad Spectrum SPF 20 Uses
- Warnings
- Directions
- CD HydraLife BB Eye Creme Enhancing Sunscreen Eye Illuminator Luminous Beige Broad Spectrum SPF 20 Other information
- Inactive ingredients
- 2 shades. Ophtalmologist-tested.
- Product Labels
FULL PRESCRIBING INFORMATION
CD HydraLife BB Eye Creme Enhancing Sunscreen Eye Illuminator Luminous Beige Broad Spectrum SPF 20
Active ingredients
Titanium Dioxide 3.65 %
Purpose
Sunscreen
CD HydraLife BB Eye Creme Enhancing Sunscreen Eye Illuminator Luminous Beige Broad Spectrum SPF 20 Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only.
Do not use
on damaged or broken skin.
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
rash occurs.
Keep out of reach of children.
If swallowed, get medical help or contact Poison Control Center right away.
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses.
- Children under 6 months of age: ask a doctor.
CD HydraLife BB Eye Creme Enhancing Sunscreen Eye Illuminator Luminous Beige Broad Spectrum SPF 20 Other information
- Protect the product in this container from excessive heat and direct sun.
Inactive ingredients
AQUA (WATER) - GLYCERIN - PHENYL TRIMETHICONE - METHYL TRIMETHICONE - ISODODECANE - DIPHENYLSILOXY PHENYL TRIMETHICONE - CETYL PEG/PPG-10/1 DIMETHICONE - MICA - BUTYLENE GLYCOL - CAPRYLIC/CAPRIC TRIGLYCERIDE - SILICA - PROPYLENE GLYCOL DICAPRYLATE/DICAPRATE - BETAINE - SORBITAN SESQUIOLEATE - DIMETHICONE/PHENYL VINYL DIMETHICONE CROSSPOLYMER - PHENOXYETHANOL - DIMETHICONE - CAPRYLYL GLYCOL - DISTEARDIMONIUM HECTORITE - ALUMINUM HYDROXIDE - SODIUM CITRATE - STEARIC ACID - SODIUM MYRISTOYL GLUTAMATE - TOCOPHERYL ACETATE - PROPYLENE CARBONATE - GLUCOSYL HESPERIDIN - NYLON 6/12 - SODIUM CHLORIDE - TERMINALIA SERICEA (TERMINALIA SERICEA BARK/ROOT EXTRACT) - PARFUM (FRAGRANCE) - ROSA HYBRID FLOWER EXTRACT - CITRIC ACID - TROMETHAMINE - BHT - CENTELLA ASIATICA LEAF EXTRACT - HYALURONIC ACID - MALVA SYLVESTRIS (MALLOW) EXTRACT - SODIUM HYALURONATE - LIMONENE - AJUGA TURKESTANICA EXTRACT - TOCOPHEROL. [MAY CONTAIN:
CI 77491, CI 77492, CI 77499 (IRON OXIDES) - CI 77891 (TITANIUM DIOXIDE)].
2 shades. Ophtalmologist-tested.
MADE IN FRANCE Parfums Christian Dior 33, avenue Hoche 75008 Paris - France In USA: 19E57 NY. Ny 10022 www.dior.com
HYDRATION AND BEAUTY CD HYDRA LIFE BB EYE CREME ENHANCING SUNSCREEN EYE ILLUMINATOR LUMINOUS BEIGE BROAD SPECTRUM SPF 20 Dior 6 ml 0.20 FL.OZ.
Product Labels


CD HydraLife BB Eye Creme Enhancing Sunscreen Eye Illuminator Luminous Beige Broad Spectrum SPF 20TITANIUM DIOXIDE CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||