Caverject
Pharmacia and Upjohn Company
Pfizer Inc
Caverject alprostadil for injection
FULL PRESCRIBING INFORMATION: CONTENTS*
- CAVERJECT DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATION AND USAGE
- CAVERJECT CONTRAINDICATIONS
- PRECAUTIONS
- CAVERJECT ADVERSE REACTIONS
- OVERDOSAGE
- CAVERJECT DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PATIENT INSTRUCTIONS FORCaverject alprostadil for injection
- PRINCIPAL DISPLAY PANEL - 20 mcg Vial Carton
- PRINCIPAL DISPLAY PANEL - 40 mcg Vial Label
- PRINCIPAL DISPLAY PANEL - 40 mcg Vial Carton
FULL PRESCRIBING INFORMATION
For Intracavernosal Use
CAVERJECT DESCRIPTION
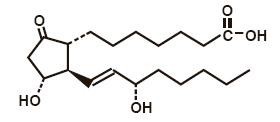
CAVERJECT Sterile Powder contains alprostadil as the naturally occurring form of prostaglandin E1 (PGE1) and is designated chemically as (11α,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid. The molecular weight is 354.49.
Alprostadil is a white to off-white crystalline powder with a melting point between 115° and 116° C. Its solubility at 35° C is 8000 micrograms per 100 milliliter double distilled water. CAVERJECT is available as a sterile freeze-dried powder for intracavernosal use in four sizes: 5, 10, 20 and 40 micrograms per vial — When reconstituted as directed with 1 milliliter of bacteriostatic water for injection or sterile water, both preserved with benzyl alcohol 0.945% w/v, gives 1.13 milliliters of reconstituted solution. Each milliliter of CAVERJECT contains 5.4, 10.5, 20.5 or 41.1 micrograms of alprostadil depending on vial strength, 172 milligrams of lactose, 47 micrograms of sodium citrate and 8.4 milligrams of benzyl alcohol. The deliverable amount of alprostadil is 5, 10, 20 or 40 micrograms per milliliter because approximately 0.4 microgram for the 5 microgram strength, 0.5 microgram for the 10 and 20 microgram strengths and 1.1 microgram for the 40 microgram strength is lost due to adsorption to the vial and syringe. When necessary, the pH of alprostadil for injection was adjusted with hydrochloric acid and/or sodium hydroxide before lyophilization.
The structural formula of alprostadil is represented below:
CLINICAL PHARMACOLOGY
Alprostadil has a wide variety of pharmacological actions; vasodilation and inhibition of platelet aggregation are among the most notable of these effects. In most animal species tested, alprostadil relaxed retractor penis and corpus cavernosum urethrae in vitro. Alprostadil also relaxed isolated preparations of human corpus cavernosum and spongiosum, as well as cavernous arterial segments contracted by either noradrenaline or PGF2α in vitro. In pigtail monkeys (Macaca nemestrina), alprostadil increased cavernous arterial blood flow in vivo. The degree and duration of cavernous smooth muscle relaxation in this animal model was dose-dependent.
Alprostadil induces erection by relaxation of trabecular smooth muscle and by dilation of cavernosal arteries. This leads to expansion of lacunar spaces and entrapment of blood by compressing the venules against the tunica albuginea, a process referred to as the corporal veno-occlusive mechanism.
Pharmacokinetics
Absorption
For the treatment of erectile dysfunction, alprostadil is administered by injection into the corpora cavernosa. The absolute bioavailability of alprostadil has not been determined.
Distribution
Following intracavernosal injection of 20 micrograms alprostadil, mean peripheral plasma concentrations of alprostadil at 30 and 60 minutes after injection (89 and 102 picograms/milliliter, respectively) were not significantly greater than baseline levels of endogenous alprostadil (96 picograms/milliliter). Alprostadil is bound in plasma primarily to albumin (81% bound) and to a lesser extent α-globulin IV-4 fraction (55% bound). No significant binding to erythrocytes or white blood cells was observed.
Metabolism
Alprostadil is rapidly converted to compounds which are further metabolized prior to excretion. Following intravenous administration, approximately 80% of circulating alprostadil is metabolized in one pass through the lungs, primarily by beta- and omega-oxidation. Hence, any alprostadil entering the systemic circulation following intracavernosal injection is very rapidly metabolized. Following intracavernosal injection of 20 micrograms alprostadil, peripheral levels of the major circulating metabolite, 13,14-dihydro-15-oxo-PGE1, increased to reach a peak 30 minutes after injection and returned to pre-dose levels by 60 minutes after injection.
Excretion
The metabolites of alprostadil are excreted primarily by the kidney, with almost 90% of an administered intravenous dose excreted in urine within 24 hours post-dose. The remainder of the dose is excreted in the feces. There is no evidence of tissue retention of alprostadil or its metabolites following intravenous administration.
Pharmacokinetics in Special Populations
Geriatric
The potential effect of age on the pharmacokinetics of alprostadil has not been formally evaluated. In patients with acute respiratory distress syndrome (ARDS), the mean (± SD) pulmonary extraction of alprostadil was 72% ± 15% in 11 elderly patients aged 65 years or older (mean, 71 ± 6 years) and 65% ± 20% in 6 young patients aged 35 years or younger (mean, 28 ± 5 years).
Pediatric
Alprostadil plasma concentrations were measured in 10 neonates (gestational age of 34 weeks in 2 infants and 38 to 40 weeks in 8 infants) receiving steady-state intravenous infusions of alprostadil to treat underlying cardiac malformations. Infusion rates of alprostadil ranged from 5 to 50 (median, 45) nanograms/kilogram/minute, resulting in alprostadil plasma concentrations ranging between 22 and 530 (median, 56) picograms/milliliter. The wide range of alprostadil plasma concentrations in neonates reflects high variability in individual clearances of alprostadil in this patient population.
Gender
The potential influence of gender on the pharmacokinetics of alprostadil has not been formally studied in healthy subjects. Two studies determined the pulmonary extraction of alprostadil following intravascular administration in 23 patients with ARDS. The mean (± SD) pulmonary extraction was 66% ± 20% in 17 male patients and 69% ± 18% in 6 female patients, suggesting that the pharmacokinetics of alprostadil are not influenced by gender.
Race
The potential influence of race on the pharmacokinetics of alprostadil has not been formally evaluated.
Renal and Hepatic Insufficiency
The pharmacokinetics of alprostadil have not been formally examined in patients with renal or hepatic insufficiency.
Pulmonary Disease
The pulmonary extraction of alprostadil following intravascular administration was reduced by 15% (66 ± 3.2% vs 78 ± 2.4%) in patients with ARDS compared with a control group of patients with normal respiratory function who were undergoing cardiopulmonary bypass surgery. Pulmonary clearance was found to vary as a function of cardiac output and pulmonary intrinsic clearance in a group of 14 patients with ARDS or at risk of developing ARDS following trauma or sepsis. In this study, the extraction efficiency of alprostadil ranged from subnormal (11%) to normal (90%), with an overall mean of 67%.
Drug-Drug Interactions
The potential for pharmacokinetic drug-drug interactions between alprostadil and other agents has not been formally studied.
INDICATION AND USAGE
CAVERJECT is indicated for the treatment of erectile dysfunction due to neurogenic, vasculogenic, psychogenic, or mixed etiology.
Intracavernosal CAVERJECT may be a useful adjunct to other diagnostic tests in the diagnosis of erectile dysfunction.
CAVERJECT CONTRAINDICATIONS
CAVERJECT should not be used in patients who have a known hypersensitivity to the drug, in patients who have conditions that might predispose them to priapism, such as sickle cell anemia or trait, multiple myeloma, or leukemia, or in patients with anatomical deformation of the penis, such as angulation, cavernosal fibrosis, or Peyronie's disease. Patients with penile implants should not be treated with CAVERJECT.
CAVERJECT should not be used in women or children and is not for use in newborns.
CAVERJECT should not be used in men for whom sexual activity is inadvisable or contraindicated.
PRECAUTIONS
General Precautions
Prolonged erection (erection lasting 4 to 6 hours) and priapism (erection lasting over 6 hours) are known to occur following intracavernosal administration of vasoactive substances, including CAVERJECT. The patient should be instructed to immediately report to his physician or, if unavailable, to seek immediate medical assistance for any erection that persists for longer than 4 hours. Treatment of priapism should be according to established medical practice.
The overall incidence of penile fibrosis, including Peyronie's disease, reported in clinical studies with CAVERJECT was 3%. In one self-injection clinical study where duration of use was up to 18 months, the incidence of fibrosis was 7.8%. Regular follow-up of patients, with careful examination of the penis, is strongly recommended to detect signs of penile fibrosis. Treatment with CAVERJECT should be discontinued in patients who develop penile angulation, cavernosal fibrosis, or Peyronie's disease.
Patients on anticoagulants, such as warfarin or heparin, may have increased propensity for bleeding after intracavernosal injection.
Underlying treatable medical causes of erectile dysfunction should be diagnosed and treated prior to initiation of therapy with CAVERJECT.
The safety and efficacy of combinations of CAVERJECT and other vasoactive agents have not been systematically studied. Therefore, the use of such combinations is not recommended.
The patient should be instructed not to re-use or to share needles or syringes. As with all prescription medicines, the patient should not allow anyone else to use his medicine.
Information for the Patient
To ensure safe and effective use of CAVERJECT, the patient should be thoroughly instructed and trained in the self-injection technique before he begins intracavernosal treatment with CAVERJECT at home. The desirable dose should be established in the physician's office. The instructions for preparation of the solution of CAVERJECT should be carefully followed. Vials with precipitates or discoloration should be discarded. The reconstituted vial is designed for one use only and should be discarded after withdrawal of proper volume of the solution. The content of the reconstituted vial should not be shaken. The needle must be properly discarded after use; it must not be re-used or shared with other persons. Patient instructions for administration are included in each package of CAVERJECT.
The dose of CAVERJECT that is established in the physician's office should not be changed by the patient without consulting the physician. The patient may expect an erection to occur within 5 to 20 minutes. A standard treatment goal is to produce an erection lasting no longer than 1 hour. Generally, CAVERJECT should be used no more than 3 times per week, with at least 24 hours between each use.
Patients should be aware of possible side effects of therapy with CAVERJECT; the most frequently occurring is penile pain after injection, usually mild to moderate in severity. A potentially serious adverse reaction with intracavernosal therapy is priapism. Accordingly, the patient should be instructed to contact the physician's office immediately or, if unavailable, to seek immediate medical assistance if an erection persists for longer than 4 hours.
The patient should report any penile pain that was not present before or that increased in intensity, as well as the occurrence of nodules or hard tissue in the penis to his physician as soon as possible. As with any intravenous injection, an infection is a possibility. Patients should be instructed to report to the physician any penile redness, swelling, tenderness or curvature of the erect penis. The patient must visit the physician's office for regular checkups for assessment of the therapeutic benefit and safety of treatment with CAVERJECT.
Note: Use of intracavernosal CAVERJECT offers no protection from the transmission of sexually transmitted diseases. Individuals who use CAVERJECT should be counseled about the protective measures that are necessary to guard against the spread of sexually transmitted diseases, including the human immunodeficiency virus (HIV).
The injection of CAVERJECT can induce a small amount of bleeding at the site of injection (see ADVERSE REACTIONS section — hematoma, ecchymosis, hemorrhage at the site of injection). In patients infected with blood-borne diseases, this could increase the risk of transmission of blood-borne diseases between partners.
In clinical trials, concomitant use of agents such as antihypertensive drugs, diuretics, antidiabetic agents (including insulin), or non-steroidal anti-inflammatory drugs had no effect on the efficacy or safety of CAVERJECT.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term carcinogenicity studies have not been conducted. Rat reproductive studies indicate that alprostadil at doses of up to 0.2 milligram/kilogram/day does not adversely affect or alter rat spermatogenesis, providing a 200-fold margin of safety compared with the usual human doses. The following battery of mutagenicity assays revealed no potential for mutagenesis: bacterial mutation (Ames), alkaline elution, rat micronucleus, sister chromatid exchange, CHO/HGPRT mammalian cell forward gene mutation, and unscheduled DNA synthesis (UDS).
A 1-year irritancy study was conducted in three groups of 5 male Cynomolgus monkeys injected intracavernosally twice weekly with either vehicle or 3 or 8.25 micrograms of alprostadil per injection. An additional two groups of 6 monkeys each were injected with vehicle or with 8.25 micrograms/injection twice weekly as described previously plus they received multiple doses during weeks 44, 48, and 52. Three monkeys from each group were retained for a 4-week recovery period. There was no evidence of drug-related penile irritancy or nonpenile tissue lesions, which could be directly related to alprostadil. The irritancy which was noted for control and treated monkeys was considered to be a result of the injection procedure itself, and any lesions noted were shown to be reversible. At the end of the 4-week recovery period, the histological changes in the penis had regressed.
Pregnancy, Nursing Mothers, and Pediatric Use
CAVERJECT is not indicated for use in newborns, children, or women.
Geriatric Use
A total of 341 subjects included in clinical studies were 65 and over. No overall differences in safety and effectiveness were observed between these subjects and younger subjects, and the other reported clinical experience has not identified differences in responses between elderly and younger patients, but decreased sensitivity of some older individuals cannot be ruled out.
CAVERJECT ADVERSE REACTIONS
Local Side Effects
The following local adverse reaction information was derived from controlled and uncontrolled studies, including an uncontrolled 18-month safety study.
| Event | CAVERJECT N = 1861 |
|---|---|
| Penile pain | 37% |
| Prolonged erection | 4% |
| Penile fibrosis |
3% |
| Injection site hematoma | 3% |
| Penis disorder |
3% |
| Injection site ecchymosis | 2% |
| Penile rash | 1% |
| Penile edema | 1% |
Penile Pain
Penile pain after intracavernosal administration of CAVERJECT was reported at least once by 37% of patients in clinical studies of up to 18 months in duration. In the majority of the cases, penile pain was rated mild or moderate in intensity. Three percent of patients discontinued treatment because of penile pain. The frequency of penile pain was 2% in 294 patients who received 1 to 3 injections of placebo.
Prolonged Erection/Priapism
In clinical trials, prolonged erection was defined as an erection that lasted for 4 to 6 hours; priapism was defined as erection that lasted 6 hours or longer. The frequency of prolonged erection after intracavernosal administration of CAVERJECT was 4%, while the frequency of priapism was 0.4%. In the majority of cases, spontaneous detumescence occurred. To minimize the chances of prolonged erection or priapism, CAVERJECT should be titrated slowly to the lowest effective dose (see DOSAGE AND ADMINISTRATION section). The patient must be instructed to immediately report to his physician or, if unavailable, to seek immediate medical assistance for any erection that persists for longer than 4 hours. If priapism is not treated immediately, penile tissue damage and permanent loss of potency may result.
Hematoma/Ecchymosis
The frequency of hematoma and ecchymosis was 3% and 2%, respectively. In most cases, hematoma/ecchymosis was judged to be a complication of a faulty injection technique. Accordingly, proper instruction of the patient in self-injection is of importance to minimize the potential of hematoma/ecchymosis (see DOSAGE AND ADMINISTRATION).
The following local adverse reactions were reported by fewer than 1% of patients after injection of CAVERJECT: balanitis, injection site hemorrhage, injection site inflammation, injection site itching, injection site swelling, injection site edema, urethral bleeding, penile warmth, numbness, yeast infection, irritation, sensitivity, phimosis, pruritus, erythema, venous leak, painful erection, and abnormal ejaculation.
Systemic Adverse Events
The following systemic adverse event information was derived from controlled and uncontrolled studies, including an uncontrolled 18-month safety study.
| Body System/Reaction | CAVERJECT N = 1861 |
|---|---|
| Cardiovascular System | |
| Hypertension | 2% |
| Central Nervous System | |
| Headache | 2% |
| Dizziness | 1% |
| Musculoskeletal System | |
| Back pain | 1% |
| Respiratory System | |
| Upper respiratory infection | 4% |
| Flu syndrome | 2% |
| Sinusitis | 2% |
| Nasal congestion | 1% |
| Cough | 1% |
| Urogenital System | |
| Prostatic Disorder |
2% |
| Miscellaneous | |
| Localized pain |
2% |
| Trauma |
2% |
The following systemic events, which were reported for < 1% of patients in clinical studies, were judged by investigators to be possibly related to use of CAVERJECT: testicular pain, scrotal disorder, scrotal edema, hematuria, testicular disorder, impaired urination, urinary frequency, urinary urgency, pelvic pain, hypotension, vasodilation, peripheral vascular disorder, supraventricular extrasystoles, vasovagal reactions, hypesthesia, non-generalized weakness, diaphoresis, rash, non-application site pruritus, skin neoplasm, nausea, dry mouth, increased serum creatinine, leg cramps, and mydriasis.
Hemodynamic changes, manifested as decreases in blood pressure and increases in pulse rate, were observed during clinical studies, principally at doses above 20 micrograms and above 30 micrograms of alprostadil, respectively, and appeared to be dose-dependent. However, these changes were usually clinically unimportant; only three patients discontinued the treatment because of symptomatic hypotension.
CAVERJECT had no clinically important effect on serum or urine laboratory tests.
OVERDOSAGE
Overdosage was not observed in clinical trials with CAVERJECT. If intracavernous overdose of CAVERJECT occurs, the patient should be under medical supervision until any systemic effects have resolved and/or until penile detumescence has occurred. Symptomatic treatment of any systemic symptoms would be appropriate.
CAVERJECT DOSAGE AND ADMINISTRATION
The dose of CAVERJECT should be individualized for each patient by careful titration under supervision by the physician. In clinical studies, patients were treated with CAVERJECT in doses ranging from 0.2 to 140 micrograms; however, since 99% of patients received doses of 60 micrograms or less, doses of greater than 60 micrograms are not recommended. In general, the lowest possible effective dose should always be employed. In clinical studies, over 80% of patients experienced an erection sufficient for sexual intercourse after intracavernosal injection of CAVERJECT. A 1/2-inch, 27- to 30-gauge needle is generally recommended.
Initial Titration in Physician's Office
Erectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology
Dosage titration should be initiated at 2.5 micrograms of alprostadil. If there is a partial response, the dose may be increased by 2.5 micrograms to a dose of 5 micrograms and then in increments of 5 to 10 micrograms, depending upon erectile response, until the dose that produces an erection suitable for intercourse and not exceeding a duration of 1 hour is reached. If there is no response to the initial 2.5-microgram dose, the second dose may be increased to 7.5 micrograms, followed by increments of 5 to 10 micrograms. The patient must stay in the physician's office until complete detumescence occurs. If there is no response, then the next higher dose may be given within 1 hour. If there is a response, then there should be at least a 1-day interval before the next dose is given.
Erectile Dysfunction of Pure Neurogenic Etiology (Spinal Cord Injury)
Dosage titration should be initiated at 1.25 micrograms of alprostadil. The dose may be increased by 1.25 micrograms to a dose of 2.5 micrograms, followed by an increment of 2.5 micrograms to a dose of 5 micrograms, and then in 5-microgram increments until the dose that produces an erection suitable for intercourse and not exceeding a duration of 1 hour is reached. The patient must stay in the physician's office until complete detumescence occurs. If there is no response, then the next higher dose may be given within 1 hour. If there is a response, then there should be at least a 1-day interval before the next dose is given.
The majority of patients (56%) in one clinical study involving 579 patients were titrated to doses of greater than 5 micrograms but less than or equal to 20 micrograms. The mean dose at the end of the titration phase was 17.8 micrograms of alprostadil.
Maintenance Therapy
The first injections of CAVERJECT must be done at the physician's office by medically trained personnel. Self-injection therapy by the patient can be started only after the patient is properly instructed and well trained in the self-injection technique. The physician should make a careful assessment of the patient's skills and competence with this procedure. The intracavernosal injection must be done under sterile conditions. The site of injection is usually along the dorso-lateral aspect of the proximal third of the penis. Visible veins should be avoided. The side of the penis that is injected and the site of injection must be alternated; the injection site must be cleansed with an alcohol swab.
The dose of CAVERJECT that is selected for self-injection treatment should provide the patient with an erection that is satisfactory for sexual intercourse and that is maintained for no longer than 1 hour. If the duration of erection is longer than 1 hour, the dose of CAVERJECT should be reduced. Self-injection therapy for use at home should be initiated at the dose that was determined in the physician's office; however, dose adjustment, if required (up to 57% of patients in one clinical study), should be made only after consultation with the physician. The dose should be adjusted in accordance with the titration guidelines described above. The effectiveness of CAVERJECT for long-term use of up to 6 months has been documented in an uncontrolled, self-injection study. The mean dose of CAVERJECT at the end of 6 months was 20.7 micrograms in this study.
Careful and continuous follow-up of the patient while in the self-injection program must be exercised. This is especially true for the initial self-injections, since adjustments in the dose of CAVERJECT may be needed. The recommended frequency of injection is no more than 3 times weekly, with at least 24 hours between each dose. The reconstituted vial of CAVERJECT is intended for single use only and should be discarded after use. The user should be instructed in the proper disposal of the syringe, needle, and vial.
While on self-injection treatment, it is recommended that the patient visit the prescribing physician's office every 3 months. At that time, the efficacy and safety of the therapy should be assessed, and the dose of CAVERJECT should be adjusted, if needed.
CAVERJECT as an Adjunct to the Diagnosis of Erectile Dysfunction
In the simplest diagnostic test for erectile dysfunction (pharmacologic testing), patients are monitored for the occurrence of an erection after an intracavernosal injection of CAVERJECT. Extensions of this testing are the use of CAVERJECT as an adjunct to laboratory investigations, such as duplex or Doppler imaging, 133Xenon washout tests, radioisotope penogram, and penile arteriography, to allow visualization and assessment of penile vasculature. For any of these tests, a single dose of CAVERJECT that induces an erection with firm rigidity should be used.
General Procedure for Solution Preparation
CAVERJECT is packaged in a 5-milliliter glass vial. Bacteriostatic water for injection or sterile water, both preserved with benzyl alcohol 0.945% w/v, must be used as the diluent for reconstitution. After reconstitution with 1 milliliter of diluent, the volume of the resulting solution is 1.13 milliliters. One milliliter of this solution will contain 5.4, 10.5, 20.5 or 41.1 micrograms of alprostadil depending on vial strength, 172 milligrams of lactose, 47 micrograms of sodium citrate and 8.4 milligrams of benzyl alcohol. The deliverable amount of alprostadil is 5, 10, 20 or 40 micrograms per milliliter because approximately 0.4 microgram for the 5 microgram strength, 0.5 microgram for the 10 and 20 microgram strengths and 1.1 microgram for the 40 microgram strength is lost due to adsorption to the vial and syringe. After reconstitution, the solution of CAVERJECT should be used within 24 hours when stored at or below 25°C (77°F) and not refrigerated or frozen. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever the solution and container permit.
HOW SUPPLIED
CAVERJECT is a dry lyophilized powder and is supplied in vials containing 6.15, 11.9, 23.2 or 46.4 micrograms of alprostadil for intracavernosal administration. Store the 5, 10 and 20 microgram strengths at or below 25°C (77°F).
Store the 40 microgram strength at 2° to 8°C (36° to 46°F) until dispensed. After dispensing, the CAVERJECT 40 microgram strength may be stored at or below 25°C (77°F) for 3 months or until expiration date, whichever occurs first.
When reconstituted and used as directed, the deliverable amount of alprostadil is 5, 10, 20 or 40 micrograms, respectively. The reconstituted solution should be used within 24 hours when stored at or below 25°C (77°F) and not refrigerated or frozen. Only the accompanying diluent or bacteriostatic water for injection with benzyl alcohol should be used when reconstituting CAVERJECT.
CAVERJECT is available in the following packages:
6–5 microgram vials NDC 0009-5131-02
6–10 microgram vials NDC 0009-3778-05
6–20 microgram vials NDC 0009-3701-05
6–40 microgram vials NDC 0009-7686-04
Other available packages:
6–20 microgram vials with diluent syringes NDC 0009-3701-01
PATIENT INSTRUCTIONS FORCaverject alprostadil for injection
IMPOTENCE: CAUSES AND TREATMENTS
There are several causes of impotence, a condition known medically as erectile dysfunction. These include: medications that you may be taking for other conditions, impaired blood circulation in the penis, nerve damage, emotional problems, excessive smoking or alcohol use, use of street drugs, and hormonal imbalances. Often, impotence is due to more than one cause.
Treatments for impotence include: switching medications (if you are taking a medication that causes impotence), administration of hormones, penile injections, use of medical devices that produce an erection, surgical procedures to correct blood flow in the penis, penile implants, and psychological counseling. Your doctor has selected CAVERJECT for injection to treat your impotence. Your doctor can also discuss other available treatments. You should not stop taking any prescription medications, unless told to do so by your doctor.
USE OF CAVERJECT
CAVERJECT is injected into a specific area of the penis and should produce an erection in 5 to 20 minutes. The erection should last for about 1 hour. Generally, you should not use CAVERJECT more than 3 times a week, with at least 24 hours between uses.
Who Should Not Use CAVERJECT?
Men who have conditions that might result in long-lasting erections should not use CAVERJECT. Some of these conditions include: sickle cell anemia or trait, leukemia, and tumor of the bone marrow (multiple myeloma). Men with penile implants, or an abnormally formed penis, or who have been advised not to engage in sexual activity should not use CAVERJECT. CAVERJECT should not be used by women or children.
What Are The Risks Of Using CAVERJECT?
Erections that last more than 4 hours can cause serious and permanent damage. Call your doctor or seek professional help immediately if you still have an erection 4 hours after injection.
The most common side effect of CAVERJECT is mild to moderate pain after injection. About one-third of patients report this effect.
Call your doctor if you notice any redness, lumps, swelling, tenderness, or curving of the erect penis.
A small amount of bleeding at the injection site may occur. Tell your doctor if you have a condition or are taking a medicine that interferes with blood clotting.
NOTE: CAVERJECT offers no protection from the transmission of sexually transmitted diseases such as HIV (the virus that causes AIDS). Small amounts of bleeding at the injection site can increase the risk of transmission of blood-borne diseases between partners.
There is no approved injectable treatment using multiple drug components or "cocktails" for erectile dysfunction. Moreover, there are no data on the efficacy and safety of these combinations.
STORAGE
- Unused packs of 5, 10 and 20 microgram vials of CAVERJECT may be stored at or below 25°C (77°F). Unused packs of the 40 microgram vial may be stored at or below 25°C (77°F) for 3 months or until expiration date, whichever occurs first. Do not freeze.
- After reconstitution, the solution of CAVERJECT should be used within 24 hours when stored at or below 25°C (77°F) and not refrigerated or frozen.
- During travel, care should be taken to avoid allowing the product to freeze or be stored at temperatures above 25°C (77°F). Therefore, do not store in checked luggage during air travel or leave in a closed automobile.
ADDITIONAL INFORMATION
There is a technical leaflet discussion of CAVERJECT written for health-care professionals that your pharmacist can let you read.
More information about erectile dysfunction and its treatment is available from the National Institutes of Health (Washington, DC), the American Foundation for Urological Diseases (Baltimore, MD), or the Impotence Institute of America (Washington, DC).
PREPARING AND INJECTING CAVERJECT
You must be properly instructed and trained in the injection technique by your doctor before using CAVERJECT.
Before using CAVERJECT, talk to your doctor about what to expect when using it, possible side effects, and what to do if side effects occur. Your dose has been selected for your individual needs. Do not change your dose without consulting your doctor. If you are not sure of the volume or dose to be used, talk to your doctor or pharmacist.
Follow these instructions exactly to prepare and inject a sterile dose of CAVERJECT.
If the needle is severely bent at any time, do not use it for injecting CAVERJECT and do not attempt to straighten it prior to injecting CAVERJECT. A severely bent and restraightened needle may be predisposed to breakage. Needle breakage, with a portion of the needle remaining in the penis, has been reported and, in some cases, required hospitalization and surgical removal. If the needle is severely bent while preparing the injection, remove it from the syringe, discard, and attach a new, unused sterile needle to the syringe as described under "Prepare the Dose" below.
Use the needle, syringe, alcohol swabs, and vials only once then safely discard the supplies and any unused solution. Discard your needle/syringe, and alcohol swabs in a special container for disposal of sharp medical supplies. Ask your doctor or pharmacist where you can get these special containers.
Supplies Needed
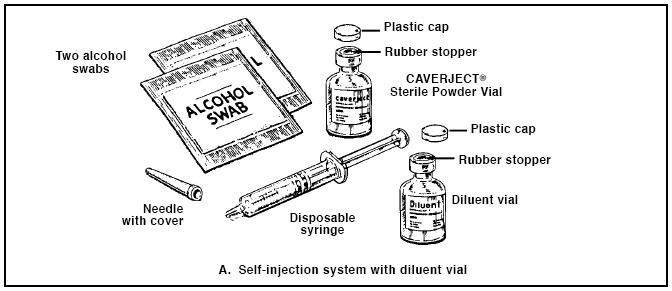
To prepare and inject CAVERJECT you will need a vial of CAVERJECT Sterile Powder, a vial of diluent (bacteriostatic water for injection or sterile water, both preserved with benzyl alcohol 0.945% w/v), a disposable sterile 3-milliliter (3-cc) syringe, a 1/2-inch 27-gauge sterile needle, and two alcohol swabs (Figure A).
CAVERJECT comes in 5, 10, 20 or 40 microgram strengths. MAKE SURE YOU HAVE THE RIGHT STRENGTH VIAL OF CAVERJECT.
Prepare the Dose
-
1. Wash your hands thoroughly, and dry them with a clean towel. -
2. Assemble the needle and syringe as follows:-
a. Remove the syringe from its sterile wrapping. -
b. To remove the sterile needle from its wrapping, carefully pull the wrapper tabs back enough to expose the sterile open end of the needle assembly. Do not touch the open end of the needle (Figure B).
-

-
-
c. While holding the sterile needle assembly between thumb and forefinger, pick up syringe with other hand. With same two fingers, remove the plastic syringe cap (Figure C). Do not touch the syringe tip.
-

-
-
d. Without removing the plastic needle cover, firmly attach the needle to the syringe tip (twist to tighten) (Figure D).
-
-
-
e. With the needle cover in place, set the syringe and needle down on a clean, level surface.
-
-
3. Remove the plastic caps from the vials of CAVERJECT and diluent. -
4. Wipe the rubber stoppers on the vials of CAVERJECT and diluent with one alcohol swab. Discard this alcohol swab. -
5. Grasp the syringe barrel (not the plunger) and remove the needle cover. Do not discard the needle cover, you will need to use it again (see step 16). Do not touch the exposed needle. Holding the syringe/needle in a straight line with the diluent vial to avoid bending the needle, push the needle through the center of the diluent vial's rubber stopper. -
6. Keeping the needle in the vial, firmly hold the vial and syringe upside down in one hand (see Figure E).
-
7. Keeping the needle tip below the level of fluid, pull back on the syringe plunger until all the diluent is removed from vial. -
8. Push the syringe plunger to the 1-cc (mL) mark on the syringe. This will expel air and excess diluent back into vial. -
9. Grasp the side of syringe barrel (not the plunger) and pull the needle/syringe from the diluent vial in a straight line to avoid bending the needle. -
10. Holding the syringe/needle in a straight line with the vial of CAVERJECT to avoid bending the needle, push the needle through the center of the rubber stopper of the vial of CAVERJECT and push the syringe plunger all the way down to expel all the diluent into the vial. Proceed immediately to step 11. -
11. Without removing the needle or touching the needle or stopper, gently swirl (do not shake) the vial until all the powder is dissolved in the diluent. Then turn the vial and needle/syringe upside down and gently swirl the vial to dissolve any powder in the neck of the vial. DO NOT USE THE SOLUTION IF IT IS CLOUDY, COLORED OR CONTAINS PARTICLES. -
12. Keeping the needle in the vial, firmly hold the vial and syringe upside down in one hand (see Figure E). -
13. Keeping the needle tip below the level of fluid, slowly pull back on the syringe plunger until all the fluid is removed from the vial. -
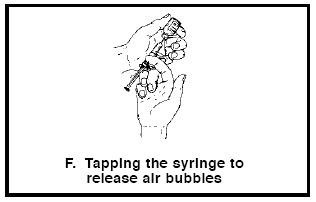
14. If there are air bubbles, gently tap the syringe barrel until they float to the top of the solution (see Figure F). Holding the syringe upright, push the syringe plunger to the correct volume mark for the dose prescribed by your doctor. This will expel any air and excess solution into the vial.
-
15. Grasp the syringe barrel (not the plunger) and pull the needle/syringe from the vial of CAVERJECT in a straight line to avoid bending the needle. -
16. Place the needle cover over the needle and set the syringe down on a level surface.
Select Injection Site
-
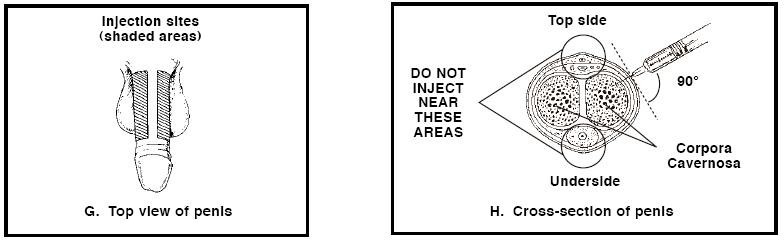
1. CAVERJECT will be injected into a corpus cavernosum (spongy tissue) of the penis. One corpus cavernosum runs the length of the right side of the penis. Another corpus cavernosum runs the length of the left side of the penis (see Figures G and H).
-
2. Choose an injection site on one side of the shaft of the penis as shown in Figure G. AVOID VISIBLE BLOOD VESSELS. -
3. WITH EACH USE OF CAVERJECT, ALTERNATE THE SIDE OF THE PENIS AND VARY THE SITE OF THE INJECTION.
Inject Your Dose of CAVERJECT
-
1. You should be sitting upright or slightly reclined when injecting CAVERJECT. -
2. If your penis is not circumcised, pull the foreskin back. Holding the head of your penis with your thumb and forefinger, stretch it lengthwise along your thigh so that you can clearly see the selected injection site. -
3. Clean the injection site with a new alcohol swab. Do not discard this swab, you will need to use it again (see step 7). -
4. Remove the cover from the needle. Reposition the penis firmly against your thigh as in step 2 to keep it from moving during the injection. -
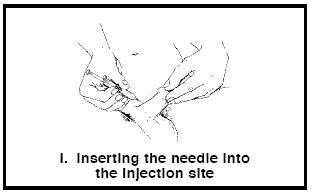
5. Hold the syringe between your thumb and index finger (Figure I). Using a steady motion, push the needle straight into the selected site until the metal part of the needle is almost entirely in the penis.
-
6. Holding the syringe barrel between two fingers, move your thumb or finger to the top of the plunger and, with a steady motion, push down on the plunger so that the entire volume of CAVERJECT is slowly injected (Figure J).

-
7. Grasp the syringe barrel and pull the needle out of your penis. APPLY PRESSURE TO THE INJECTION SITE WITH THE ALCOHOL SWAB FOR ABOUT 5 MINUTES OR UNTIL BLEEDING STOPS.
Disposal of Injection Materials
-
1. Discard your needle, syringe, and alcohol swabs in a special container for disposal of sharp medical supplies. Ask your doctor or pharmacist where you can obtain these special containers. Follow the directions on your disposal container for proper disposal procedures. -
2. Do not re-use or share needles or syringes. As with all prescription medicines, do not allow anyone else to use your medicine.
Rx only

LAB-0009-3.0
Revised September 2006
PRINCIPAL DISPLAY PANEL - 20 mcg Vial Carton
NDC 0009-3701-05
Contains 6 of NDC 0009-5133-08
Rx only
Pfizer
Caverject®
alprostadil for
injection
for intracavernosal use only
6 Single-Dose Vials
20 mcg
Diluent to be Used With This Product
Contains Benzyl Alcohol as a Preservative
This Package Does NOT Contain Diluent

PRINCIPAL DISPLAY PANEL - 40 mcg Vial Label
Pfizer
NDC 0009-7686-01
Caverject®
alprostadil for injection
40 mcg
for intracavernosal
use only
Single Dose Vial
Rx only
PRINCIPAL DISPLAY PANEL - 40 mcg Vial Carton
NDC 0009-7686-04
Contains 6 of NDC 0009-7686-01
Rx only
Pfizer
Caverject®
alprostadil for
injection
for intracavernosal use only
6 Single-Dose Vials
40 mcg
Diluent to be Used With This Product
Contains Benzyl Alcohol as a Preservative
This Package Does NOT Contain Diluent

CaverjectALPROSTADIL INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CaverjectALPROSTADIL INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CaverjectALPROSTADIL INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CaverjectALPROSTADIL INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||