Carvedilol
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use carvedilol safely and effectively. See full prescribing information for carvedilol tablets, USP. Carvedilol Tablets, USP Initial U.S. Approval: 1995 RECENT MAJOR CHANGES(5.9)5.14INDICATIONS AND USAGECarvedilol is an alpha/beta-adrenergic blocking agent indicated for the treatment of: Left ventricular dysfunction following myocardial infarction in clinically stable patients (1.2) Hypertension (1.3) DOSAGE AND ADMINISTRATIONTake with food. Individualize dosage and monitor during up-titration. (2) Left ventricular dysfunction following myocardial infarction: Start at 6.25 mg twice daily and increase to 12.5 mg then 25 mg twice daily after intervals of 3 to 10 days. A lower starting dose or slower titration may be used. (2.2) Hypertension: Start at 6.25 mg twice daily and increase if needed for blood pressure control to 12.5 mg then 25 mg twice daily over intervals of 1 to 2 weeks. (2.3) DOSAGE FORMS AND STRENGTHS(3)CONTRAINDICATIONS Bronchial asthma or related bronchospastic conditions (4) Second- or third-degree AV block (4) Sick sinus syndrome (4) Severe bradycardia (unless permanent pacemaker in place) (4) Patients in cardiogenic shock or decompensated heart failure requiring the use of IV inotropic therapy. (4) Severe hepatic impairment (2.4, 4) History of serious hypersensitivity reaction (e.g., Stevens-Johnson syndrome, anaphylactic reaction, angioedema) to any component of this medication or other medications containing carvedilol. (4) WARNINGS AND PRECAUTIONS Acute exacerbation of coronary artery disease upon cessation of therapy: Do not abruptly discontinue. (5.1) Bradycardia, hypotension, worsening heart failure/fluid retention may occur. Reduce the dose as needed. (5.2, 5.3, 5.4) Non-allergic bronchospasm (e.g., chronic bronchitis and emphysema): Avoid β-blockers. (4) However, if deemed necessary, use with caution and at lowest effective dose. (5.5) Diabetes: Monitor glucose as β-blockers may mask symptoms of hypoglycemia or worsen hyperglycemia. (5.6) Side EffectsMost common adverse events (6.1): Left ventricular dysfunction following myocardial infarction (≥10%): Dizziness, fatigue, hypotension, diarrhea, hyperglycemia, asthenia, bradycardia, weight increase Hypertension (≥5%): Dizziness To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS CYP P450 2D6 enzyme inhibitors may increase and rifampin may decrease carvedilol levels. (7.1, 7.5) Hypotensive agents (e.g., reserpine, MAO inhibitors, clonidine) may increase the risk of hypotension and/or severe bradycardia. (7.2) Cyclosporine or digoxin levels may increase. (7.3, 7.4) Both digitalis glycosides and β-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia. (7.4) Amiodarone may increase carvedilol levels resulting in further slowing of the heart rate or cardiac conduction. (7.6) Verapamil- or diltiazem-type calcium channel blockers may affect ECG and/or blood pressure. (7.7) Insulin and oral hypoglycemics action may be enhanced. (7.8)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 CARVEDILOL INDICATIONS AND USAGE
- 2 CARVEDILOL DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CARVEDILOL CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Cessation of Therapy
- 5.2 Bradycardia
- 5.3 Hypotension
- 5.4 Heart Failure/Fluid Retention
- 5.5 Non-allergic Bronchospasm
- 5.6 Glycemic Control in Type 2 Diabetes
- 5.7 Peripheral Vascular Disease
- 5.8 Deterioration of Renal Function
- 5.9 Major Surgery
- 5.10 Thyrotoxicosis
- 5.11 Pheochromocytoma
- 5.12 Prinzmetal’s Variant Angina
- 5.13 Risk of Anaphylactic Reaction
- 5.14 Intraoperative Floppy Iris Syndrome
- 6 CARVEDILOL ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 CARVEDILOL DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- 17.2 FDA-Approved Patient Labeling
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 3.125 mg
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 6.25 mg
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 12.5 mg
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 25 mg
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.2 Left Ventricular Dysfunction Following Myocardial Infarction
[see Clinical Studies (14.2)]
1.3 Hypertension
[see Clinical Studies (14.3, 14.4)][see Drug Interactions (7.2)]
2 DOSAGE AND ADMINISTRATION
2.2 Left Ventricular Dysfunction Following Myocardial Infarction
2.3 Hypertension
2.4 Hepatic Impairment
[see Contraindications (4)]
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
- Bronchial asthma or related bronchospastic conditions. Deaths from status asthmaticus have been reported following single doses of carvedilol tablets.
- Second- or third-degree AV block
- Sick sinus syndrome
- Severe bradycardia (unless a permanent pacemaker is in place)
- Patients with cardiogenic shock or who have decompensated heart failure requiring the use of intravenous inotropic therapy. Such patients should first be weaned from intravenous therapy before initiating carvedilol tablets.
- Patients with severe hepatic impairment
- Patients with a history of a serious hypersensitivity reaction (e.g., Stevens-Johnson syndrome, anaphylactic reaction, angioedema) to any component of this medication or other medications containing carvedilol.
5 WARNINGS AND PRECAUTIONS
5.1 Cessation of Therapy
Patients with coronary artery disease, who are being treated with carvedilol, should be advised against abrupt discontinuation of therapy. Severe exacerbation of angina and the occurrence of myocardial infarction and ventricular arrhythmias have been reported in angina patients following the abrupt discontinuation of therapy with β-blockers. The last 2 complications may occur with or without preceding exacerbation of the angina pectoris. As with other β-blockers, when discontinuation of carvedilol is planned, the patients should be carefully observed and advised to limit physical activity to a minimum. Carvedilol should be discontinued over 1 to 2 weeks whenever possible. If the angina worsens or acute coronary insufficiency develops, it is recommended that carvedilol be promptly reinstituted, at least temporarily. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue therapy with carvedilol abruptly even in patients treated only for hypertension or heart failure.
5.2 Bradycardia
5.3 Hypotension
[see Dosage and Administration (2.2, 2.3)]
5.4 Heart Failure/Fluid Retention
[see Dosage and Administration (2)]
5.5 Non-allergic Bronchospasm
5.6 Glycemic Control in Type 2 Diabetes
[see Clinical Studies (14.4)].
5.7 Peripheral Vascular Disease
5.8 Deterioration of Renal Function
5.9 Major Surgery
the
5.10 Thyrotoxicosis
5.11 Pheochromocytoma
5.12 Prinzmetal’s Variant Angina
5.13 Risk of Anaphylactic Reaction
5.14 Intraoperative Floppy Iris Syndrome
progressive
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Left Ventricular Dysfunction Following Myocardial Infarction:
Hypertension:
| Carvedilol | Placebo | |
|---|---|---|
| (n = 1,142) | (n = 462) | |
|
*Shown are events with rate >1% rounded to nearest integer. |
||
|
Cardiovascular
|
||
| Bradycardia |
2 |
— |
| Postural hypotension |
2 |
— |
| Peripheral edema |
1 |
— |
|
Central Nervous System
|
||
| Dizziness |
6 |
5 |
| Insomnia |
2 |
1 |
|
Gastrointestinal
|
||
| Diarrhea |
2 |
1 |
|
Hematologic
|
||
| Thrombocytopenia |
1 |
— |
|
Metabolic
|
||
| Hypertriglyceridemia |
1 |
— |
Incidence >0.1% to ≤1%
Cardiovascular:
Central and Peripheral Nervous System:
Gastrointestinal: [see Adverse Reactions (6.2)]
Psychiatric:
Respiratory System: [see Contraindications (4)]
Reproductive, male:
Skin and Appendages:
Special Senses:
Urinary System:
Autonomic Nervous System:
Metabolic and Nutritional:
Hematologic:
6.2 Laboratory Abnormalities
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CYP2D6 Inhibitors and Poor Metabolizers
[see Clinical Pharmacology (12.3)]
7.2 Hypotensive Agents
7.3 Cyclosporine
7.4 Digitalis Glycosides
[see Clinical Pharmacology (12.5)]
7.5 Inducers/Inhibitors of Hepatic Metabolism
[see Clinical Pharmacology (12.5)]. max [see Clinical Pharmacology (12.5)]
7.6 Amiodarone
[see Clinical Pharmacology (12.5)]
7.7 Calcium Channel Blockers
7.8 Insulin or Oral Hypoglycemics
[see Warnings and Precautions (5.6)]
7.9 Anesthesia
[see Overdosage (10)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
22222
8.3 Nursing Mothers
2
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
for excessive bradycardia:
to support cardiovascular function:
11 DESCRIPTION
1
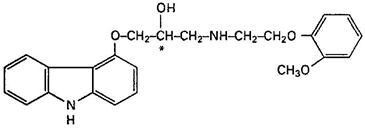
242624
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
1
12.2 Pharmacodynamics
Left Ventricular Dysfunction Following Myocardial Infarction:
Hypertension:
1
1[see Dosage and Administration (2)]
12.3 Pharmacokinetics
12.4 Specific Populations
Geriatric:
Hepatic Impairment:
Renal Impairment:
12.5 Drug-Drug Interactions
Amiodarone:[see Drug Interactions (7.6)]
Cimetidine:max [see Drug Interactions (7.5) ]
Digoxin:[see Drug Interactions (7.4)]
Glyburide:
Hydrochlorothiazide:
Rifampin:max [see Drug Interactions (7.5)]
Torsemide:
Warfarin:
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
22
in vitroin vivo
22
14 CLINICAL STUDIES
14.2 Left Ventricular Dysfunction Following Myocardial Infarction
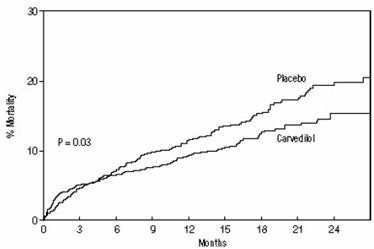
Figure 1. Survival Analysis for CAPRICORN (intent-to-treat)
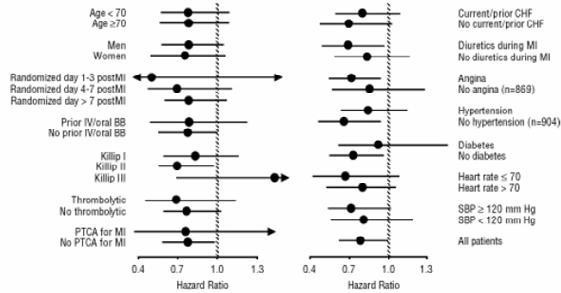
Figure 2. Effects on Mortality for Subgroups in CAPRICORN
14.3 Hypertension
[see Adverse Reactions (6)]
14.4 Hypertension With Type 2 Diabetes Mellitus
In a double-blind study (GEMINI), carvedilol, added to an ACE inhibitor or angiotensin receptor blocker, was evaluated in a population with mild-to-moderate hypertension and well-controlled type 2 diabetes mellitus. The mean HbA1c at baseline was 7.2%. Carvedilol was titrated to a mean dose of 17.5 mg twice daily and maintained for 5 months. Carvedilol had no adverse effect on glycemic control, based on HbA1c measurements (mean change from baseline of 0.02%, 95% CI -0.06 to 0.1, p = NS) [see Warnings and Precautions (5.6)].
16 HOW SUPPLIED/STORAGE AND HANDLING
Carvedilol Tablets USP, 3.125 mg
| Bottles of 30 |
NDC 54868-1980-0 |
| Bottles of 60 |
NDC 54868-1980-1 |
Carvedilol Tablets USP, 6.25 mg
| Bottles of 30 |
NDC 54868-3062-0 |
| Bottles of 60 |
NDC 54868-3062-1 |
| Bottles of 90 |
NDC 54868-3062-2 |
| Bottles of 180 |
NDC 54868-3062-3 |
Carvedilol Tablets USP, 12.5 mg
| Bottles of 30 |
NDC 54868-5773-1 |
| Bottles of 60 |
NDC 54868-5773-0 |
| Bottles of 90 |
NDC 54868-5773-2 |
| Bottles of 180 |
NDC 54868-5773-3 |
Carvedilol Tablets USP, 25 mg
| Bottles of 30 |
NDC 54868-5817-1 |
| Bottles of 60 |
NDC 54868-5817-0 |
| Bottles of 90 |
NDC 54868-5817-2 |
| Bottles of 180 |
NDC 54868-5817-3 |
Store at
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (17.2).
17.1 Patient Advice
- Patients should take carvedilol with food.
- Patients should not interrupt or discontinue using carvedilol without a physician’s advice.
- Patients should consult their physician if they experience signs or symptoms of worsening heart failure such as weight gain or increasing shortness of breath.
- Patients may experience a drop in blood pressure when standing, resulting in dizziness and, rarely, fainting. Patients should sit or lie down when these symptoms of lowered blood pressure occur.
- If experiencing dizziness or fatigue, patients should avoid driving or hazardous tasks.
- Patients should consult a physician if they experience dizziness or faintness, in case the dosage should be adjusted.
- Diabetic patients should report any changes in blood sugar levels to their physician.
- Contact lens wearers may experience decreased lacrimation.
17.2 FDA-Approved Patient Labeling
PATIENT INFORMATION
Carvedilol Tablets, USP
What are carvedilol tablets?
- To treat patients who had a heart attack that worsened how well the heart pumps
- To treat patients with high blood pressure (hypertension)
Who should not take carvedilol tablets?
- Have severe heart failure and are hospitalized in the intensive care unit or require certain intravenous medications that help support circulation (inotropic medications)
- Are prone to asthma or other breathing problems
- Have a slow heartbeat or a heart that skips a beat (irregular heartbeat)
- Have liver problems
- Are allergic to any of the ingredients in carvedilol tablets. The active ingredient is carvedilol. See the end of this leaflet for a list of all the ingredients in carvedilol tablets.
What should I tell my doctor before taking carvedilol tablets?
- Have asthma or other lung problems (such as bronchitis or emphysema)
- Have problems with blood flow in your feet and legs (peripheral vascular disease) carvedilol tablets can make some of your symptoms worse.
- Have diabetes
- Have thyroid problems
- Have a condition called pheochromocytoma
- Have had severe allergic reactions
- Are pregnant or trying to become pregnant. It is not known if carvedilol tablets are safe for your unborn baby. You and your doctor should talk about the best way to control your high blood pressure during pregnancy.
- Are breastfeeding. It is not known if carvedilol passes into your breast milk. You should not breastfeed while using carvedilol tablets.
- Are scheduled for surgery and will be given anesthetic agents
- Are scheduled for cataract surgery and have taken or are currently taking carvedilol tablets.
- Are taking prescription or non-prescription medicines, vitamins, and herbal supplements. Carvedilol tablets and certain other medicines can affect each other and cause serious side effects. Carvedilol tablets may affect the way other medicines work. Also, other medicines may affect how well carvedilol tablets work.
How should I take carvedilol tablets?
It is important for you to take your medicine every day as directed by your doctor. If you stop taking carvedilol tablets suddenly, you could have chest pain and/or a heart attack. If your doctor decides that you should stop taking carvedilol tablets, your doctor may slowly lower your dose over a period of time before stopping them completely.
- Take carvedilol tablets exactly as prescribed. Your doctor will tell you how many tablets to take and how often. In order to minimize possible side effects, your doctor might begin with a low dose and then slowly increase the dose.
- Do not stop taking carvedilol tablets and do not change the amount of carvedilol tablets you take without talking to your doctor.
- Tell your doctor if you gain weight or have trouble breathing while taking carvedilol tablets.
- Take carvedilol tablets with food.
- If you miss a dose of carvedilol tablets, take your dose as soon as you remember, unless it is time to take your next dose. Take your next dose at the usual time. Do not take 2 doses at the same time.
- If you take too much carvedilol, call your doctor or poison control center right away.
What should I avoid while taking carvedilol tablets?
What are possible side effects of carvedilol tablets?
- Low blood pressure (which may cause dizziness or fainting when you stand up). If these happen, sit or lie down right away and tell your doctor.
- Tiredness. If you feel tired or dizzy you should not drive, use machinery, or do anything that needs you to be alert.
- Slow heartbeat.
- Changes in your blood sugar. If you have diabetes, tell your doctor if you have any changes in your blood sugar levels.
- Carvedilol tablets may hide some of the symptoms of low blood sugar, especially a fast heartbeat.
- Carvedilol tablets may mask the symptoms of hyperthyroidism (overactive thyroid).
- Worsening of severe allergic reactions.
- Rare but serious allergic reactions (including hives or swelling of the face, lips, tongue, and/or throat that may cause difficulty in breathing or swallowing) have happened in patients who were on carvedilol tablets. These reactions can be life-threatening.
How should I store carvedilol tablets?
- Store carvedilol tablets at 20 to 25°C (68 to 77°F). Keep the tablets dry.
- Safely, throw away carvedilol tablets that are out of date or no longer needed.
- Keep carvedilol tablets and all medicines out of the reach of children.
General information about carvedilol tablets
What are the ingredients in carvedilol tablets?
What is high blood pressure (hypertension)?
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
Relabeling and Repackaging by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 3.125 mg
Carvedilol Tablets, USP
3.125 mg
Pharmacist: Dispense the accompanying
Patient Information Leaflet to each patient.
Rx only

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 6.25 mg
Carvedilol Tablets, USP
6.25 mg
Pharmacist: Dispense the accompanying
Patient Information Leaflet to each patient.
Rx only

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 12.5 mg
Carvedilol Tablets, USP
12.5 mg
Pharmacist: Dispense the accompanying
Patient Information Leaflet to each patient.
Rx only

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 25 mg
Carvedilol Tablets, USP
25 mg
Pharmacist: Dispense the accompanying
Patient Information Leaflet to each patient.
Rx only

CarvedilolCarvedilol TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CarvedilolCarvedilol TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CarvedilolCarvedilol TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CarvedilolCarvedilol TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!