Carvedilol
These highlights do not include all the information needed to use carvedilol safely and effectively. See full prescribing information for carvedilol tablets, USP.Carvedilol Tablets, USP Initial U.S. Approval: 1995
FULL PRESCRIBING INFORMATION: CONTENTS*
- Recent Major Changes Section
- Indications & Usage Section
- Dosage & Administration Section
- Dosage Forms & Strengths Section
- Contraindications Section
- Warnings and Precautions Section
- Side Effects Section
- Drug Interactions Section
- Use In Specific Populations Section
- Overdosage Section
- Description Section
- Clinical Pharmacology Section
- Nonclinical Toxicology Section
- Clinical Studies Section
- How Supplied Section
- Information For Patients Section
- SPL PATIENT PACKAGE INSERT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
Indications & Usage Section
[see Clinical Studies (14.2)]
[see Clinical Studies (14.3, 14.4)][see Drug Interactions (7.2)].
Dosage & Administration Section
[see Contraindications (4)]
Dosage Forms & Strengths Section
Contraindications Section
- Bronchial asthma or related bronchospastic conditions. Deaths from status asthmaticus have been reported following single doses of carvedilol tablets.
- Second- or third-degree AV block
- Sick sinus syndrome
- Severe bradycardia (unless a permanent pacemaker is in place)
- Patients with cardiogenic shock or who have decompensated heart failure requiring the use of intravenous inotropic therapy. Such patients should first be weaned from intravenous therapy before initiating carvedilol tablets.
- Patients with severe hepatic impairment
- Patients with a history of a serious hypersensitivity reaction (e.g., Stevens-Johnson syndrome, anaphylactic reaction, angioedema) to any component of this medication or other medications containing carvedilol.
Warnings and Precautions Section
Patients with coronary artery disease, who are being treated with carvedilol, should be advised against abrupt discontinuation of therapy. Severe exacerbation of angina and the occurrence of myocardial infarction and ventricular arrhythmias have been reported in angina patients following the abrupt discontinuation of therapy with β-blockers. The last 2 complications may occur with or without preceding exacerbation of the angina pectoris. As with other β-blockers, when discontinuation of carvedilol is planned, the patients should be carefully observed and advised to limit physical activity to a minimum. Carvedilol should be discontinued over 1 to 2 weeks whenever possible. If the angina worsens or acute coronary insufficiency develops, it is recommended that carvedilol be promptly reinstituted, at least temporarily. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue therapy with carvedilol abruptly even in patients treated only for hypertension or heart failure.
[see Dosage and Administration (2.2, 2.3)]
[see Dosage and Administration (2)]
[see Clinical Studies (14.4)].
the
progressive
Side Effects Section
Left Ventricular Dysfunction Following Myocardial Infarction:
Hypertension:
| Carvedilol | Placebo | |
|---|---|---|
| (n = 1,142) | (n = 462) | |
|
Cardiovascular
|
||
| Bradycardia |
2 |
— |
| Postural hypotension |
2 |
— |
| Peripheral edema |
1 |
— |
|
Central Nervous System
|
||
| Dizziness |
6 |
5 |
| Insomnia |
2 |
1 |
|
Gastrointestinal
|
||
| Diarrhea |
2 |
1 |
|
Hematologic
|
||
| Thrombocytopenia |
1 |
— |
|
Metabolic
|
||
| Hypertriglyceridemia |
1 |
— |
Incidence >0.1% to ≤1%
Cardiovascular:
Central and Peripheral Nervous System:
Gastrointestinal: [see Adverse Reactions (6.2)]
Psychiatric:
Respiratory System: [see Contraindications (4)]
Reproductive, male:
Skin and Appendages:
Special Senses:
Urinary System:
Autonomic Nervous System:
Metabolic and Nutritional:
Hematologic:
Drug Interactions Section
[see Clinical Pharmacology (12.3)]
[see Clinical Pharmacology (12.5)]
[see Clinical Pharmacology (12.5)]. max [see Clinical Pharmacology (12.5)]
[see Clinical Pharmacology (12.5)]
[see Warnings and Precautions (5.6)]
[see Overdosage (10)]
Use In Specific Populations Section
22222
2
Overdosage Section
for excessive bradycardia:
to support cardiovascular function:
Description Section

Clinical Pharmacology Section
1
Left Ventricular Dysfunction Following Myocardial Infarction:
Hypertension:
1
1[see Dosage and Administration (2)]
Geriatric:
Hepatic Impairment:
Renal Impairment:
Amiodarone:[see Drug Interactions (7.6)]
Cimetidine:max [see Drug Interactions (7.5) ]
Digoxin:[see Drug Interactions (7.4)]
Glyburide:
Hydrochlorothiazide:
Rifampin:max [see Drug Interactions (7.5)]
Torsemide:
Warfarin:
Nonclinical Toxicology Section
22
in vitroin vivo
22
Clinical Studies Section

Figure 1. Survival Analysis for CAPRICORN (intent-to-treat)
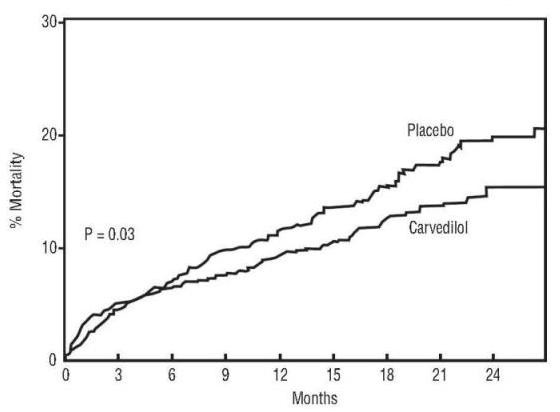
Figure 2. Effects on Mortality for Subgroups in CAPRICORN
[see Adverse Reactions (6)]
In a double-blind study (GEMINI), carvedilol, added to an ACE inhibitor or angiotensin receptor blocker, was evaluated in a population with mild-to-moderate hypertension and well-controlled type 2 diabetes mellitus. The mean HbA1c at baseline was 7.2%. Carvedilol was titrated to a mean dose of 17.5 mg twice daily and maintained for 5 months. Carvedilol had no adverse effect on glycemic control, based on HbA1c measurements (mean change from baseline of 0.02%, 95% CI -0.06 to 0.1, p = NS) [see Warnings and Precautions (5.6)].
How Supplied Section
Carvedilol Tablets USP, 3.125 mg
Carvedilol Tablets USP, 6.25 mg
Carvedilol Tablets USP, 12.5 mg
Carvedilol Tablets USP, 25 mg
Store at
Information For Patients Section
See FDA-Approved Patient Labeling (17.2).
- Patients should take carvedilol with food.
- Patients should not interrupt or discontinue using carvedilol without a physician’s advice.
- Patients should consult their physician if they experience signs or symptoms of worsening heart failure such as weight gain or increasing shortness of breath.
- Patients may experience a drop in blood pressure when standing, resulting in dizziness and, rarely, fainting. Patients should sit or lie down when these symptoms of lowered blood pressure occur.
- If experiencing dizziness or fatigue, patients should avoid driving or hazardous tasks.
- Patients should consult a physician if they experience dizziness or faintness, in case the dosage should be adjusted.
- Diabetic patients should report any changes in blood sugar levels to their physician.
- Contact lens wearers may experience decreased lacrimation.
SPL PATIENT PACKAGE INSERT
PATIENT INFORMATION
Carvedilol Tablets, USP
What are carvedilol tablets?
- To treat patients who had a heart attack that worsened how well the heart pumps
- To treat patients with high blood pressure (hypertension)
Who should not take carvedilol tablets?
- Have severe heart failure and are hospitalized in the intensive care unit or require certain intravenous medications that help support circulation (inotropic medications)
- Are prone to asthma or other breathing problems
- Have a slow heartbeat or a heart that skips a beat (irregular heartbeat)
- Have liver problems
- Are allergic to any of the ingredients in carvedilol tablets. The active ingredient is carvedilol. See the end of this leaflet for a list of all the ingredients in carvedilol tablets.
What should I tell my doctor before taking carvedilol tablets?
- Have asthma or other lung problems (such as bronchitis or emphysema)
- Have problems with blood flow in your feet and legs (peripheral vascular disease) carvedilol tablets can make some of your symptoms worse.
- Have diabetes
- Have thyroid problems
- Have a condition called pheochromocytoma
- Have had severe allergic reactions
- Are pregnant or trying to become pregnant. It is not known if carvedilol tablets are safe for your unborn baby. You and your doctor should talk about the best way to control your high blood pressure during pregnancy.
- Are breastfeeding. It is not known if carvedilol passes into your breast milk. You should not breastfeed while using carvedilol tablets.
- Are scheduled for surgery and will be given anesthetic agents
- Are scheduled for cataract surgery and have taken or are currently taking carvedilol tablets.
- Are taking prescription or non-prescription medicines, vitamins, and herbal supplements. Carvedilol tablets and certain other medicines can affect each other and cause serious side effects. Carvedilol tablets may affect the way other medicines work. Also, other medicines may affect how well carvedilol tablets work.
How should I take carvedilol tablets?
It is important for you to take your medicine every day as directed by your doctor. If you stop taking carvedilol tablets suddenly, you could have chest pain and/or a heart attack. If your doctor decides that you should stop taking carvedilol tablets, your doctor may slowly lower your dose over a period of time before stopping them completely.
- Take carvedilol tablets exactly as prescribed. Your doctor will tell you how many tablets to take and how often. In order to minimize possible side effects, your doctor might begin with a low dose and then slowly increase the dose.
- Do not stop taking carvedilol tablets and do not change the amount of carvedilol tablets you take without talking to your doctor.
- Tell your doctor if you gain weight or have trouble breathing while taking carvedilol tablets.
- Take carvedilol tablets with food.
- If you miss a dose of carvedilol tablets, take your dose as soon as you remember, unless it is time to take your next dose. Take your next dose at the usual time. Do not take 2 doses at the same time.
- If you take too much carvedilol, call your doctor or poison control center right away.
What should I avoid while taking carvedilol tablets?
What are possible side effects of carvedilol tablets?
- Low blood pressure (which may cause dizziness or fainting when you stand up). If these happen, sit or lie down right away and tell your doctor.
- Tiredness. If you feel tired or dizzy you should not drive, use machinery, or do anything that needs you to be alert.
- Slow heartbeat.
- Changes in your blood sugar. If you have diabetes, tell your doctor if you have any changes in your blood sugar levels.
- Carvedilol tablets may hide some of the symptoms of low blood sugar, especially a fast heartbeat.
- Carvedilol tablets may mask the symptoms of hyperthyroidism (overactive thyroid).
- Worsening of severe allergic reactions.
- Rare but serious allergic reactions (including hives or swelling of the face, lips, tongue, and/or throat that may cause difficulty in breathing or swallowing) have happened in patients who were on carvedilol tablets. These reactions can be life-threatening.
How should I store carvedilol tablets?
- Store carvedilol tablets at 20 to 25°C (68 to 77°F). Keep the tablets dry.
- Safely, throw away carvedilol tablets that are out of date or no longer needed.
- Keep carvedilol tablets and all medicines out of the reach of children.
General information about carvedilol tablets
What are the ingredients in carvedilol tablets?
What is high blood pressure (hypertension)?
Aurolife Pharma LLC
Aurobindo Pharma USA, Inc.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

CarvedilolCarvedilol TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!