Carbidopa and Levodopa
NCS HealthCare of KY, Inc dba Vangard Labs
Carbidopa and Levodopa ER Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- CARBIDOPA AND LEVODOPA DESCRIPTION
- CLINICAL PHARMACOLOGY
- CARBIDOPA AND LEVODOPA INDICATIONS AND USAGE
- CARBIDOPA AND LEVODOPA CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CARBIDOPA AND LEVODOPA ADVERSE REACTIONS
- OVERDOSAGE
- CARBIDOPA AND LEVODOPA DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
CARBIDOPA AND LEVODOPA DESCRIPTION
Carbidopa and levodopa extended-release tablets are for the treatment of Parkinson's disease and syndrome.
Carbidopa, USP, an inhibitor of aromatic amino acid decarboxylation, is a white, crystalline compound, slightly soluble in water, with a molecular weight of 244.25. It is designated chemically as(-)-L-alpha-hydrazino-alpha-methyl-beta-(3,4-dihydroxybenzene) propanoic acid monohydrate. Its structural formula is:
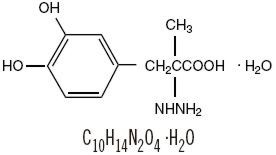
Tablet content is expressed in terms of anhydrous carbidopa, which has a molecular weight of 226.23.
Levodopa, USP, an aromatic amino acid, is a white, crystalline compound, slightly soluble in water, with a molecular weight of 197.19. It is designated chemically as (-)-L-alpha-amino- beta-(3,4-dihydroxybenzene) propanoic acid. Its structural formula is:
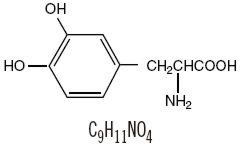
Each extended-release tablet, for oral administration, contains either 25 mg of carbidopa and 100 mg of levodopa or 50 mg of carbidopa and 200 mg of levodopa. In addition, each tablet contains the following inactive ingredients: FD&C Blue No. 2 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, hydroxypropyl cellulose, hypromellose, and magnesium stearate.
Carbidopa and levodopa extended-release tablets are designed in a drug delivery system that controls the release of carbidopa and levodopa as the tablets slowly erode. The 25 mg/100 mg carbidopa and levodopa extended-release tablet is available to facilitate titration and as an alternative to the half-tablet of 50 mg/200 mg carbidopa and levodopa extended-release.
CLINICAL PHARMACOLOGY
Mechanism of Action
Parkinson's disease is a progressive, neurodegenerative disorder of the extrapyramidal nervous system affecting the mobility and control of the skeletal muscular system. Its characteristic features include resting tremor, rigidity, and bradykinetic movements. Symptomatic treatments, such as levodopa therapies, may permit the patient better mobility.
Current evidence indicates that symptoms of Parkinson's disease are related to depletion of dopamine in the corpus striatum. Administration of dopamine is ineffective in the treatment of Parkinson's disease apparently because it does not cross the blood-brain barrier. However, levodopa, the metabolic precursor of dopamine, does cross the blood-brain barrier, and presumably is converted to dopamine in the brain. This is thought to be the mechanism whereby levodopa relieves symptoms of Parkinson's disease.
Pharmacodynamics
When levodopa is administered orally it is rapidly decarboxylated to dopamine in extracerebral tissues so that only a small portion of a given dose is transported unchanged to the central nervous system. For this reason, large doses of levodopa are required for adequate therapeutic effect and these may often be accompanied by nausea and other adverse reactions, some of which are attributable to dopamine formed in extracerebral tissues.
Since levodopa competes with certain amino acids for transport across the gut wall, the absorption of levodopa may be impaired in some patients on a high protein diet.
Carbidopa inhibits decarboxylation of peripheral levodopa. It does not cross the blood-brain barrier and does not affect the metabolism of levodopa within the central nervous system.
Since its decarboxylase inhibiting activity is limited to extracerebral tissues, administration of carbidopa with levodopa makes more levodopa available for transport to the brain.
Patients treated with levodopa therapy for Parkinson's disease may develop motor fluctuations characterized by end-of-dose failure, peak dose dyskinesia, and akinesia. The advanced form of motor fluctuations (‘on-off’ phenomenon) is characterized by unpredictable swings from mobility to immobility. Although the causes of the motor fluctuations are not completely understood, in some patients they may be attenuated by treatment regimens that produce steady plasma levels of levodopa.
Carbidopa and levodopa extended-release tablets contain either 25 mg of carbidopa and 100 mg of levodopa or 50 mg of carbidopa and 200 mg of levodopa, in a sustained-release dosage form designed to release these ingredients over a 4 to 6 hour period. With carbidopa and levodopa extended-release there is less variation in plasma levodopa levels than with carbidopa and levodopa immediate-release, the conventional formulation. However, carbidopa and levodopa extended-release is less systemically bioavailable than carbidopa and levodopa immediate-release and may require increased daily doses to achieve the same level of symptomatic relief as provided by carbidopa and levodopa immediate-release.
In clinical trials, patients with moderate to severe motor fluctuations who received carbidopa and levodopa extended-release did not experience quantitatively significant reductions in ‘off’ time when compared to carbidopa and levodopa immediate-release. However, global ratings of improvement as assessed by both patient and physician were better during therapy with carbidopa and levodopa extended-release than with carbidopa and levodopa immediate-release. In patients without motor fluctuations, carbidopa and levodopa extended-release under controlled conditions, provided the same therapeutic benefit with less frequent dosing when compared to carbidopa and levodopa immediate-release.
Pharmacokinetics
Carbidopa reduces the amount of levodopa required to produce a given response by about 75% and, when administered with levodopa, increases both plasma levels and the plasma half-life of levodopa, and decreases plasma and urinary dopamine and homovanillic acid.
Elimination half-life of levodopa in the presence of carbidopa is about 1.5 hours. Following carbidopa and levodopa extended-release, the apparent half-life of levodopa may be prolonged because of continuous absorption.
In healthy elderly subjects (56 to 67 years old) the mean time-to-peak concentration of levodopa after a single dose of 50 mg/200 mg carbidopa and levodopa extended-release was about 2 hours as compared to 0.5 hours after standard carbidopa and levodopa immediate-release. The maximum concentration of levodopa after a single dose of carbidopa and levodopa extended-release was about 35% of the standard carbidopa and levodopa immediate-release (1151 vs. 3256 ng/mL). The extent of availability of levodopa from carbidopa and levodopa extended-release was about 70% to 75% relative to intravenous levodopa or standard carbidopa and levodopa immediate-release in the elderly. The absolute bioavailability of levodopa from carbidopa and levodopa extended-release (relative to I.V.) in young subjects was shown to be only about 44%. The extent of availability and the peak concentrations of levodopa were comparable in the elderly after a single dose and at steady-state after t.i.d. administration of 50 mg/200 mg carbidopa and levodopa extended-release. In elderly subjects, the average trough levels of levodopa at steady-state after the extended-release tablet were about 2-fold higher than after the standard carbidopa and levodopa immediate-release (163 vs. 74 ng/mL).
In these studies, using similar total daily doses of levodopa, plasma levodopa concentrations with carbidopa and levodopa extended-release fluctuated in a narrower range than with carbidopa and levodopa immediate-release. Because the bioavailability of levodopa from carbidopa and levodopa extended-release relative to carbidopa and levodopa immediate-release is approximately 70% to 75%, the daily dosage of levodopa necessary to produce a given clinical response with the extended-release formulation will usually be higher.
The extent of availability and peak concentrations of levodopa after a single dose of 50 mg/200 mg carbidopa and levodopa extended-release increased by about 50% and 25%, respectively, when administered with food.
At steady-state, the bioavailability of carbidopa from carbidopa and levodopa immediate-release tablets is approximately 99% relative to the concomitant administration of carbidopa and levodopa. At steady-state, carbidopa bioavailability from 50 mg/200 mg carbidopa and levodopa extended-release is approximately 58% relative to that from carbidopa and levodopa immediate-release.
Pyridoxine hydrochloride (vitamin B6), in oral doses of 10 mg to 25 mg, may reverse the effects of levodopa by increasing the rate of aromatic amino acid decarboxylation. Carbidopa inhibits this action of pyridoxine.
CARBIDOPA AND LEVODOPA INDICATIONS AND USAGE
Carbidopa and levodopa extended-release tablets are indicated in the treatment of the symptoms of idiopathic Parkinson's disease (paralysis agitans), postencephalitic parkinsonism, and symptomatic parkinsonism which may follow injury to the nervous system by carbon monoxide intoxication and/or manganese intoxication.
CARBIDOPA AND LEVODOPA CONTRAINDICATIONS
Nonselective MAO inhibitors are contraindicated for use with carbidopa and levodopa extended-release tablets. These inhibitors must be discontinued at least 2 weeks prior to initiating therapy with carbidopa and levodopa extended-release. Carbidopa and levodopa extended-release may be administered concomitantly with the manufacturer's recommended dose of an MAO inhibitor with selectivity for MAO type B (e.g., selegiline hydrochloride) (see PRECAUTIONS: Drug Interactions).
Carbidopa and levodopa extended-release is contraindicated in patients with known hypersensitivity to any component of this drug and in patients with narrow-angle glaucoma.
Because levodopa may activate a malignant melanoma, carbidopa and levodopa extended-release should not be used in patients with suspicious, undiagnosed skin lesions or a history of melanoma.
WARNINGS
When patients are receiving levodopa without a decarboxylase inhibitor, levodopa must be discontinued at least 12 hours before carbidopa and levodopa extended-release is started. In order to reduce adverse reactions, it is necessary to individualize therapy. Carbidopa and levodopa extended-release should be substituted at a dosage that will provide approximately 25% of the previous levodopa dosage (see DOSAGE AND ADMINISTRATION).
Carbidopa does not decrease adverse reactions due to central effects of levodopa. By permitting more levodopa to reach the brain, particularly when nausea and vomiting is not a dose-limiting factor, certain adverse CNS effects, e.g., dyskinesias, will occur at lower dosages and sooner during therapy with carbidopa and levodopa extended-release than with levodopa alone.
Patients receiving carbidopa and levodopa extended-release may develop increased dyskinesias compared to carbidopa and levodopa immediate-release. Dyskinesias are a common side effect of carbidopa-levodopa treatment. The occurrence of dyskinesias may require dosage reduction.
As with levodopa, carbidopa and levodopa extended-release may cause mental disturbances. These reactions are thought to be due to increased brain dopamine following administration of levodopa. All patients should be observed carefully for the development of depression with concomitant suicidal tendencies. Patients with past or current psychoses should be treated with caution.
Carbidopa and levodopa extended-release should be administered cautiously to patients with severe cardiovascular or pulmonary disease, bronchial asthma, renal, hepatic or endocrine disease.
As with levodopa, care should be exercised in administering carbidopa and levodopa extended-release to patients with a history of myocardial infarction who have residual atrial, nodal, or ventricular arrhythmias. In such patients, cardiac function should be monitored with particular care during the period of initial dosage adjustment, in a facility with provisions for intensive cardiac care.
As with levodopa, treatment with carbidopa and levodopa extended-release may increase the possibility of upper gastrointestinal hemorrhage in patients with a history of peptic ulcer.
Neuroleptic Malignant Syndrome (NMS)
Sporadic cases of a symptom complex resembling NMS have been reported in association with dose reductions or withdrawal of carbidopa and levodopa immediate-release and carbidopa and levodopa extended-release.
Therefore, patients should be observed carefully when the dosage of carbidopa and levodopa extended-release is reduced abruptly or discontinued, especially if the patient is receiving neuroleptics.
NMS is an uncommon but life threatening syndrome characterized by fever or hyperthermia. Neurological findings, including muscle rigidity, involuntary movements, altered consciousness, mental status changes; other disturbances, such as autonomic dysfunction, tachycardia, tachypnea, sweating, hyper- or hypotension; laboratory findings, such as creatine phosphokinase elevation, leukocytosis, myoglobinuria, and increased serum myoglobin have been reported.
The early diagnosis of this condition is important for the appropriate management of these patients. Considering NMS as a possible diagnosis and ruling out other acute illnesses (e.g., pneumonia, systemic infection, etc.) is essential. This may be especially complex if the clinical presentation includes both serious medical illness and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system (CNS) pathology.
The management of NMS should include: 1) intensive symptomatic treatment and medical monitoring and 2) treatment of any concomitant serious medical problems for which specific treatments are available. Dopamine agonists, such as bromocriptine, and muscle relaxants, such as dantrolene, are often used in the treatment of NMS; however, their effectiveness has not been demonstrated in controlled studies.
PRECAUTIONS
General
As with levodopa, periodic evaluations of hepatic, hematopoietic, cardiovascular, and renal function are recommended during extended therapy.
Patients with chronic wide-angle glaucoma may be treated cautiously with carbidopa and levodopa extended-release provided the intraocular pressure is well controlled and the patient is monitored carefully for changes in intraocular pressure during therapy.
Dopaminergic agents, including levodopa, may be associated with somnolence and very rarely episodes of sudden onset of sleep. In some cases, these episodes may occur without awareness or warning during daily activities. Patients must be informed of this and advised to exercise caution while driving or operating machines while being treated with dopaminergic agents, including levodopa. Patients who have experienced somnolence and/or an episode of sudden sleep onset must refrain from driving or operating machines (see Information for Patients).
Melanoma
Epidemiological studies have shown that patients with Parkinson’s disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson’s disease or other factors, such as drugs used to treat Parkinson’s disease, is unclear.
For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using carbidopa and levodopa extended-release for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
Information for Patients
The patient should be informed that carbidopa and levodopa extended-release tablets are a sustained-release formulation of carbidopa and levodopa which releases these ingredients over a 4 to 6 hour period. It is important that carbidopa and levodopa extended-release be taken at regular intervals according to the schedule outlined by the physician. The patient should be cautioned not to change the prescribed dosage regimen and not to add any additional antiparkinson medications, including other carbidopa and levodopa preparations, without first consulting the physician.
If abnormal involuntary movements appear or get worse during treatment with carbidopa and levodopa extended-release, the physician should be notified, as dosage adjustment may be necessary.
Patients should be advised that sometimes the onset of effect of the first morning dose of carbidopa and levodopa extended-release may be delayed for up to one hour compared with the response usually obtained from the first morning dose of carbidopa and levodopa immediate-release. The physician should be notified if such delayed responses pose a problem in treatment.
Patients should be advised that, occasionally, dark color (red, brown, or black) may appear in saliva, urine, or sweat after ingestion of carbidopa and levodopa extended-release. Although the color appears to be clinically insignificant, garments may become discolored.
The patient should be informed that a change in diet to foods that are high in protein may delay the absorption of levodopa and may reduce the amount taken up in the circulation. Excessive acidity also delays stomach emptying, thus delaying the absorption of levodopa. Iron salts (such as in multi-vitamin tablets) may also reduce the amount of levodopa available to the body. The above factors may reduce the clinical effectiveness of the levodopa or carbidopa-levodopa therapy.
Patients must be advised that the whole or half tablet should be swallowed without chewing or crushing.
Patients should be alerted to the possibility of sudden onset of sleep during daily activities, in some cases without awareness or warning signs, when they are taking dopaminergic agents, including levodopa. Patients should be advised to exercise caution while driving or operating machinery and that if they have experienced somnolence and/or sudden sleep onset, they must refrain from these activities (see PRECAUTIONS: General).
There have been reports of patients experiencing intense urges to gamble, increased sexual urges, and other intense urges, and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone and that are generally used for the treatment of Parkinson’s disease, including carbidopa and levodopa extended-release. Although it is not proven that the medications caused these events, these urges were reported to have stopped in some cases when the dose was reduced or the medication was stopped. Prescribers should ask patients about the development of new or increased gambling urges, sexual urges or other urges while being treated with carbidopa and levodopa extended-release. Patients should inform their physician if they experience new or increased gambling urges, increased sexual urges, or other intense urges while taking carbidopa and levodopa extended-release. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking carbidopa and levodopa extended-release.
NOTE: The suggested advice to patients being treated with carbidopa and levodopa extended-release tablets is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
Laboratory Tests
Abnormalities in laboratory tests may include elevations of liver function tests such as alkaline phosphatase, SGOT (AST), SGPT (ALT), lactic dehydrogenase, and bilirubin. Abnormalities in blood urea nitrogen and positive Coombs test have also been reported. Commonly, levels of blood urea nitrogen, creatinine, and uric acid are lower during administration of carbidopa and levodopa preparations than with levodopa.
Carbidopa and levodopa preparations, such as carbidopa and levodopa immediate-release and carbidopa and levodopa extended-release, may cause a false-positive reaction for urinary ketone bodies when a test tape is used for determination of ketonuria. This reaction will not be altered by boiling the urine specimen. False-negative tests may result with the use of glucose-oxidase methods of testing for glucosuria.
Cases of falsely diagnosed pheochromocytoma in patients on carbidopa-levodopa therapy have been reported very rarely. Caution should be exercised when interpreting the plasma and urine levels of catecholamines and their metabolites in patients on levodopa or carbidopa-levodopa therapy.
Drug Interactions
Caution should be exercised when the following drugs are administered concomitantly with carbidopa and levodopa extended-release.
Symptomatic postural hypotension has occurred when carbidopa and levodopa preparations were added to the treatment of patients receiving some antihypertensive drugs. Therefore, when therapy with carbidopa and levodopa extended-release is started, dosage adjustment of the antihypertensive drug may be required.
For patients receiving monoamine oxidase (MAO) inhibitors (Type A or B), see CONTRAINDICATIONS. Concomitant therapy with selegiline and carbidopa-levodopa may be associated with severe orthostatic hypotension not attributable to carbidopa-levodopa alone (see CONTRAINDICATIONS).
There have been rare reports of adverse reactions, including hypertension and dyskinesia, resulting from the concomitant use of tricyclic antidepressants and carbidopa and levodopa preparations.
Dopamine D2 receptor antagonists (e.g., phenothiazines, butyrophenones, risperidone) and isoniazid may reduce the therapeutic effects of levodopa. In addition, the beneficial effects of levodopa in Parkinson's disease have been reported to be reversed by phenytoin and papaverine. Patients taking these drugs with carbidopa and levodopa extended-release should be carefully observed for loss of therapeutic response.
Iron salts may reduce the bioavailability of levodopa and carbidopa. The clinical relevance is unclear.
Although metoclopramide may increase the bioavailability of levodopa by increasing gastric emptying, metoclopramide may also adversely affect disease control by its dopamine receptor antagonistic properties.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year bioassay of carbidopa and levodopa immediate-release, no evidence of carcinogenicity was found in rats receiving doses of approximately 2 times the maximum daily human dose of carbidopa and 4 times the maximum daily human dose of levodopa (equivalent to 8 carbidopa and levodopa extended-release tablets).
In reproduction studies with carbidopa and levodopa immediate-release, no effects on fertility were found in rats receiving doses of approximately 2 times the maximum daily human dose of carbidopa and 4 times the maximum daily human dose of levodopa (equivalent to 8 carbidopa and levodopa extended-release tablets).
Pregnancy
Teratogenic Effects
Pregnancy Category C
No teratogenic effects were observed in a study in mice receiving up to 20 times the maximum recommended human dose of carbidopa and levodopa immediate-release. There was a decrease in the number of live pups delivered by rats receiving approximately 2 times the maximum recommended human dose of carbidopa and approximately 5 times the maximum recommended human dose of levodopa during organogenesis. Carbidopa and levodopa immediate-release caused both visceral and skeletal malformations in rabbits at all doses and ratios of carbidopa/levodopa tested, which ranged from 10 times/5 times the maximum recommended human dose of carbidopa/levodopa to 20 times/10 times the maximum recommended human dose of carbidopa/levodopa.
There are no adequate or well controlled studies in pregnant women. It has been reported from individual cases that levodopa crosses the human placental barrier, enters the fetus, and is metabolized. Carbidopa concentrations in fetal tissue appeared to be minimal. Use of carbidopa and levodopa extended-release in women of childbearing potential requires that the anticipated benefits of the drug be weighed against possible hazards to mother and child.
Nursing Mothers
In a study of one nursing mother with Parkinson’s disease, excretion of levodopa in human breast milk was reported. Therefore, caution should be exercised when carbidopa and levodopa extended-release is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Use of the drug in patients below the age of 18 is not recommended.
CARBIDOPA AND LEVODOPA ADVERSE REACTIONS
In controlled clinical trials, patients predominantly with moderate to severe motor fluctuations while on carbidopa and levodopa immediate-release were randomized to therapy with either carbidopa and levodopa immediate-release or carbidopa and levodopa extended-release. The adverse experience frequency profile of carbidopa and levodopa extended-release did not differ substantially from that of carbidopa and levodopa immediate-release, as shown in Table 1.
| Adverse Experience |
Carbidopa and Levodopa Extended-release n=491 % |
Carbidopa and Levodopa Immediate-release n=524 % |
| Dyskinesia | 16.5 | 12.2 |
| Nausea | 5.5 | 5.7 |
| Hallucinations | 3.9 | 3.2 |
| Confusion | 3.7 | 2.3 |
| Dizziness | 2.9 | 2.3 |
| Depression | 2.2 | 1.3 |
| Urinary tract infection | 2.2 | 2.3 |
| Headache | 2 | 1.9 |
| Dream abnormalities | 1.8 | 0.8 |
| Dystonia | 1.8 | 0.8 |
| Vomiting | 1.8 | 1.9 |
| Upper respiratory infection | 1.8 | 1 |
| Dyspnea | 1.6 | 0.4 |
| ‘On-Off’ phenomena | 1.6 | 1.1 |
| Back pain | 1.6 | 0.6 |
| Dry mouth | 1.4 | 1.1 |
| Anorexia | 1.2 | 1.1 |
| Diarrhea | 1.2 | 0.6 |
| Insomnia | 1.2 | 1 |
| Orthostatic hypotension | 1 | 1.1 |
| Shoulder pain | 1 | 0.6 |
| Chest pain | 1 | 0.8 |
| Muscle cramps | 0.8 | 1 |
| Paresthesia | 0.8 | 1.1 |
| Urinary frequency | 0.8 | 1.1 |
| Dyspepsia | 0.6 | 1.1 |
| Constipation | 0.2 | 1.5 |
Abnormal laboratory findings occurring at a frequency of 1% or greater in approximately 443 patients who received carbidopa and levodopa extended-release and 475 who received carbidopa and levodopa immediate-release during controlled clinical trials included: decreased hemoglobin and hematocrit; elevated serum glucose; white blood cells, bacteria and blood in the urine.
The adverse experiences observed in patients in uncontrolled studies were similar to those seen in controlled clinical studies.
Other adverse experiences reported overall in clinical trials in 748 patients treated with carbidopa and levodopa extended-release, listed by body system in order of decreasing frequency, include:
Body as a Whole: Asthenia, fatigue, abdominal pain, orthostatic effects.
Cardiovascular: Palpitation, hypertension, hypotension, myocardial infarction.
Gastrointestinal: Gastrointestinal pain, dysphagia, heartburn.
Metabolic: Weight loss.
Musculoskeletal: Leg pain.
Nervous System/Psychiatric: Chorea, somnolence, falling, anxiety, disorientation, decreased mental acuity, gait abnormalities, extrapyramidal disorder, agitation, nervousness, sleep disorders, memory impairment.
Respiratory: Cough, pharyngeal pain, common cold.
Skin: Rash.
Special Senses: Blurred vision.
Urogenital: Urinary incontinence.
Laboratory Tests: Decreased white blood cell count and serum potassium; increased BUN, serum creatinine and serum LDH; protein and glucose in the urine.
The following adverse experiences have been reported in post-marketing experience with carbidopa and levodopa extended-release:
Cardiovascular: Cardiac irregularities, syncope.
Gastrointestinal: Taste alterations, dark saliva.
Hypersensitivity: Angioedema, urticaria, pruritus, bullous lesions (including pemphigus-like reactions).
Nervous System/Psychiatric: Neuroleptic malignant syndrome (NMS), (see WARNINGS), increased tremor, peripheral neuropathy, psychotic episodes including delusions and paranoid ideation, pathological gambling, increased libido including hypersexuality, impulse control symptoms.
Skin: Alopecia, flushing, dark sweat.
Urogenital: Dark urine.
Other adverse reactions that have been reported with levodopa alone and with various carbidopa-levodopa formulations and may occur with carbidopa and levodopa extended-release are:
Cardiovascular: Phlebitis.
Gastrointestinal: Gastrointestinal bleeding, development of duodenal ulcer, sialorrhea, bruxism, hiccups, flatulence, burning sensation of tongue.
Hematologic: Hemolytic and nonhemolytic anemia, thrombocytopenia, leukopenia, agranulocytosis.
Hypersensitivity: Henoch-Schonlein purpura.
Metabolic: Weight gain, edema.
Nervous System/Psychiatric: Ataxia, depression with suicidal tendencies, dementia, euphoria, convulsions (however, a causal relationship has not been established); bradykinetic episodes, numbness, muscle twitching, blepharospasm (which may be taken as an early sign of excess dosage; consideration of dosage reduction may be made at this time), trismus, activation of latent Horner's syndrome, nightmares.
Skin: Malignant melanoma (see also CONTRAINDICATIONS), increased sweating.
Special Senses: Oculogyric crisis, mydriasis, diplopia.
Urogenital: Urinary retention, priapism.
Miscellaneous: Faintness, hoarseness, malaise, hot flashes, sense of stimulation, bizarre breathing patterns.
Laboratory Tests: Abnormalities in alkaline phosphatase, SGOT (AST), SGPT (ALT), bilirubin, Coombs test, uric acid.
OVERDOSAGE
Management of acute overdosage with carbidopa and levodopa extended-release is the same as with levodopa. Pyridoxine is not effective in reversing the actions of carbidopa and levodopa extended-release.
General supportive measures should be employed, along with immediate gastric lavage. Intravenous fluids should be administered judiciously and an adequate airway maintained. Electrocardiographic monitoring should be instituted and the patient carefully observed for the development of arrhythmias; if required, appropriate antiarrhythmic therapy should be given. The possibility that the patient may have taken other drugs as well as carbidopa and levodopa extended-release should be taken into consideration. To date, no experience has been reported with dialysis; hence, its value in overdosage is not known.
Based on studies in which high doses of levodopa and/or carbidopa were administered, a significant proportion of rats and mice given single oral doses of levodopa of approximately 1500 to 2000 mg/kg are expected to die. A significant proportion of infant rats of both sexes are expected to die at a dose of 800 mg/kg. A significant proportion of rats are expected to die after treatment with similar doses of carbidopa. The addition of carbidopa in a 1:10 ratio with levodopa increases the dose at which a significant proportion of mice are expected to die to 3360 mg/kg.
CARBIDOPA AND LEVODOPA DOSAGE AND ADMINISTRATION
Carbidopa and levodopa extended-release tablets contain carbidopa and levodopa in a 1:4 ratio as either the 50 mg/200 mg tablet or the 25 mg/100 mg tablet. The daily dosage of carbidopa and levodopa extended-release tablets must be determined by careful titration. Patients should be monitored closely during the dose adjustment period, particularly with regard to appearance or worsening of involuntary movements, dyskinesias or nausea. Carbidopa and levodopa extended-release tablets should not be chewed or crushed.
Standard drugs for Parkinson’s disease, other than levodopa without a decarboxylase inhibitor, may be used concomitantly while carbidopa and levodopa extended-release tablets are being administered, although their dosage may have to be adjusted.
Since carbidopa prevents the reversal of levodopa effects caused by pyridoxine, carbidopa and levodopa extended-release tablets can be given to patients receiving supplemental pyridoxine (vitamin B6).
Initial Dosage
Patients Currently Treated with Conventional Carbidopa-Levodopa Preparations
Studies show that peripheral dopa-decarboxylase is saturated by the bioavailable carbidopa at doses of 70 mg a day and greater. Because the bioavailabilities of carbidopa and levodopa in carbidopa and levodopa immediate-release tablets and carbidopa and levodopa extended-release tablets are different, appropriate adjustments should be made, as shown in Table 2.
| Tablet | Amount of Levodopa (mg) in Each Tablet |
Approximate Bioavailability |
Approximate Amount of Bioavailable Levodopa (mg) in Each Tablet |
| Carbidopa and Levodopa Extended-release Tablets 50 mg/200 mg |
200 | 0.70 to 0.75 |
140 to 150 |
| Carbidopa and Levodopa Immediate-release Tablets 25 mg/100 mg |
100 | 0.99 |
99 |
Dosage with carbidopa and levodopa extended-release tablets should be substituted at an amount that provides approximately 10% more levodopa per day, although this may need to be increased to a dosage that provides up to 30% more levodopa per day depending on clinical response (see DOSAGE AND ADMINISTRATION: Titration with Carbidopa and Levodopa Extended-release Tablets). The interval between doses of carbidopa and levodopa extended-release tablets should be 4 to 8 hours during the waking day (see CLINICAL PHARMACOLOGY: Pharmacodynamics).
A guideline for initiation of carbidopa and levodopa extended-release tablets is shown in Table 3.
| Carbidopa and Levodopa Immediate-release Tablets | Carbidopa and Levodopa Extended-release Tablets |
Total Daily Dose |
Suggested Dosage Regimen |
| 300–400 | 200 mg b.i.d. |
| 500–600 | 300 mg b.i.d. or 200 mg t.i.d. |
| 700–800 | A total of 800 mg in 3 or more divided doses (e.g., 300 mg a.m., 300 mg early p.m. and 200 mg later p.m.) |
| 900–1000 | A total of 1000 mg in 3 or more divided doses (e.g., 400 mg a.m., 400 mg early p.m., and 200 mg later p.m.) |
Patients Currently Treated with Levodopa Without a Decarboxylase Inhibitor
Levodopa must be discontinued at least 12 hours before therapy with carbidopa and levodopa extended-release tablets is started. Carbidopa and levodopa extended-release tablets should be substituted at a dosage that will provide approximately 25% of the previous levodopa dosage. In patients with mild to moderate disease, the initial dose is usually one tablet of 50 mg/200 mg carbidopa and levodopa extended-release tablets b.i.d.
Patients Not Receiving Levodopa
In patients with mild to moderate disease, the initial recommended dose is one tablet of 50 mg/200 mg carbidopa and levodopa extended-release tablets b.i.d. Initial dosage should not be given at intervals of less than 6 hours.
Titration with Carbidopa and Levodopa Extended-release Tablets
Following initiation of therapy, doses and dosing intervals may be increased or decreased depending upon therapeutic response. Most patients have been adequately treated with doses of carbidopa and levodopa extended-release tablets that provide 400 mg to 1600 mg of levodopa per day, administered as divided doses at intervals ranging from 4 to 8 hours during the waking day. Higher doses of carbidopa and levodopa extended-release tablets (2400 mg or more of levodopa per day) and shorter intervals (less than 4 hours) have been used, but are not usually recommended.
When doses of carbidopa and levodopa extended-release tablets are given at intervals of less than 4 hours, and/or if the divided doses are not equal, it is recommended that the smaller doses be given at the end of the day.
An interval of at least 3 days between dosage adjustments is recommended.
Maintenance
Because Parkinson's disease is progressive, periodic clinical evaluations are recommended; adjustment of the dosage regimen of carbidopa and levodopa extended-release tablets may be required.
Addition of Other Antiparkinson Medications
Anticholinergic agents, dopamine agonists, and amantadine can be given with carbidopa and levodopa extended-release tablets. Dosage adjustment of carbidopa and levodopa extended-release tablets may be necessary when these agents are added.
A dose of carbidopa and levodopa immediate-release tablets 25 mg/100 mg or 10 mg/100 mg (one half or a whole tablet) can be added to the dosage regimen of carbidopa and levodopa extended-release tablets in selected patients with advanced disease who need additional immediate-release levodopa for a brief time during daytime hours.
Interruption of Therapy
Sporadic cases of a symptom complex resembling Neuroleptic Malignant Syndrome (NMS) have been associated with dose reductions and withdrawal of carbidopa and levodopa immediate-release tablets or carbidopa and levodopa extended-release tablets.
Patients should be observed carefully if abrupt reduction or discontinuation of carbidopa and levodopa extended-release tablets is required, especially if the patient is receiving neuroleptics (see WARNINGS).
If general anesthesia is required, carbidopa and levodopa extended-release tablets may be continued as long as the patient is permitted to take oral medication. If therapy is interrupted temporarily, the patient should be observed for symptoms resembling NMS, and the usual dosage should be administered as soon as the patient is able to take oral medication.
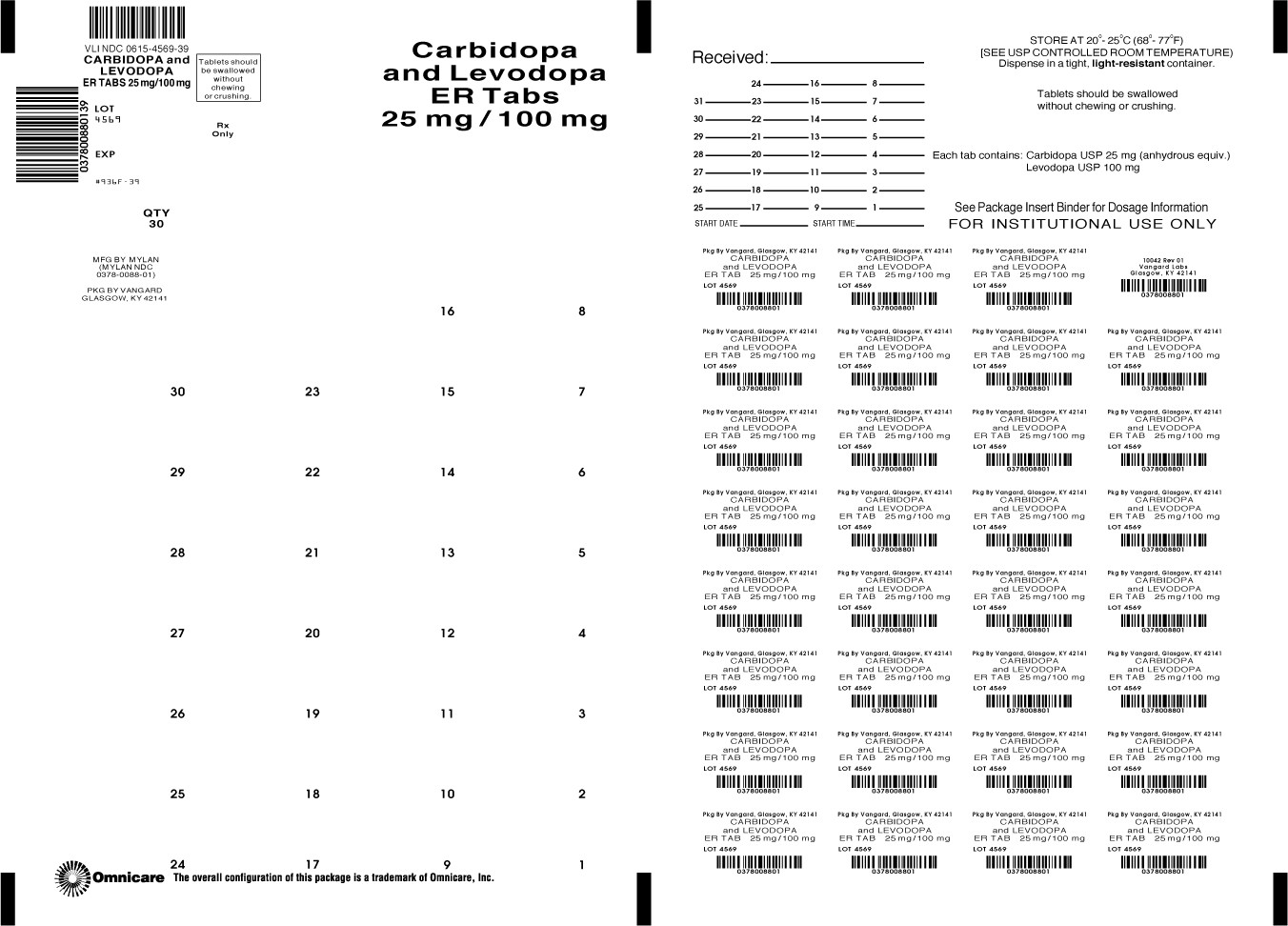
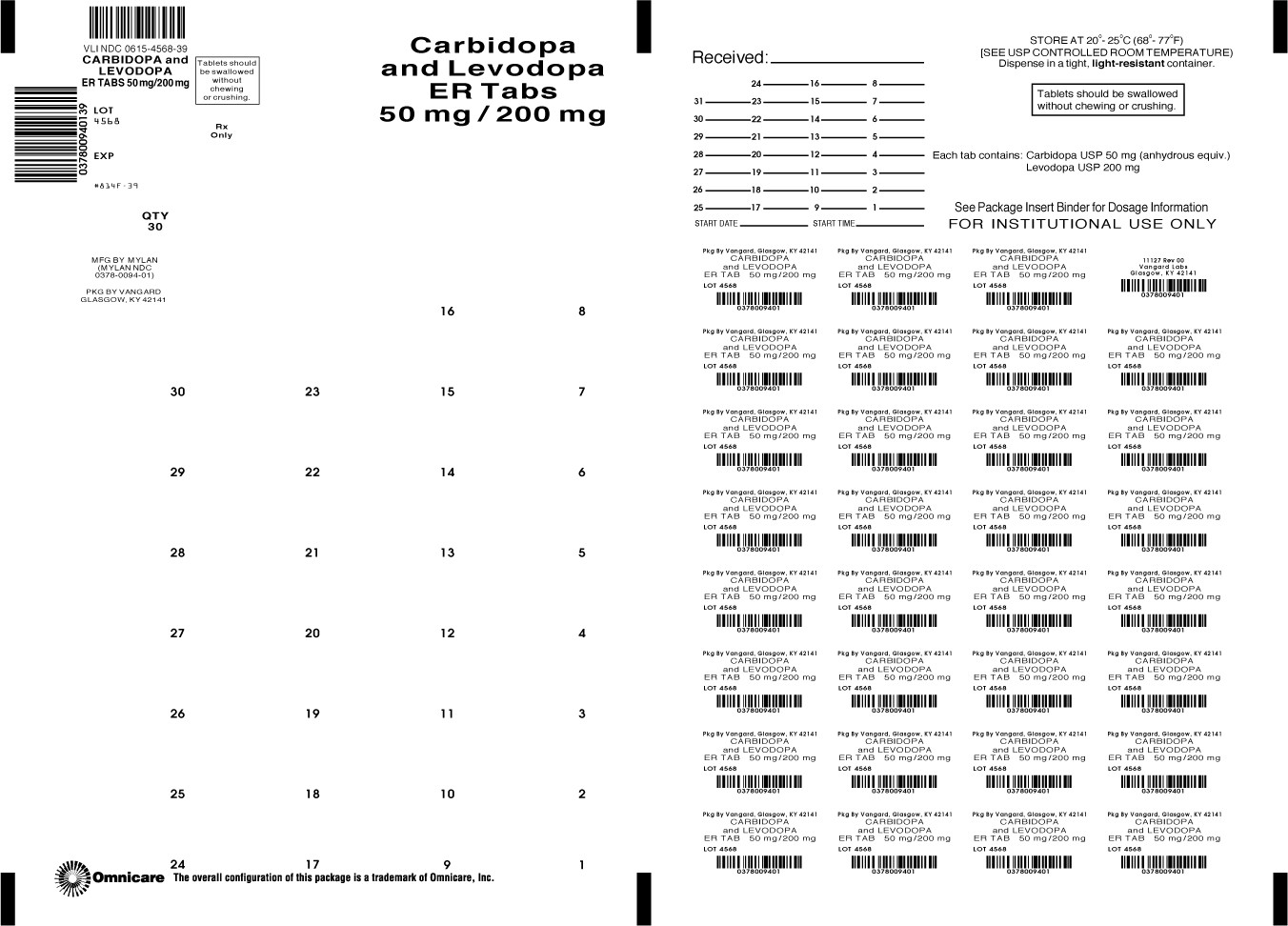
HOW SUPPLIED
Carbidopa and Levodopa Extended-release Tablets are available containing 25 mg of carbidopa, USP and 100 mg of levodopa , USP or 50 mg of carbidopa, USP and 200 mg of levodopa, USP.
The 25 mg/100 mg tablets are purple, oval, unscored tablets debossed with MYLAN on one side of the tablet and 88 on the other side of the tablet. They are available as follows:
NDC 0615-4569-39
blistercards of 30 tablets
NDC 0615-4569-31
blistercards of 31 tablets
The 50 mg/200 mg tablets are purple, oval, scored tablets debossed with MYLAN on one side of the tablet and 9 to the left of the score and 4 to the right of the score on the other side of the tablet. They are available as follows:
NDC 0615-4568-39
blistercards of 30 tablets
NDC 0615-4568-31
blistercards of 31 tablets
Dispense in a tight, light resistant container as defined in the USP using a child-resistant closure.
Keep container tightly closed.
DO NOT STORE ABOVE 30°C (86°F).
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
REVISED SEPTEMBER 2011
CBLVER:R11
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Carbidopa and LevodopaCarbidopa and Levodopa TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Carbidopa and LevodopaCarbidopa and Levodopa TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||