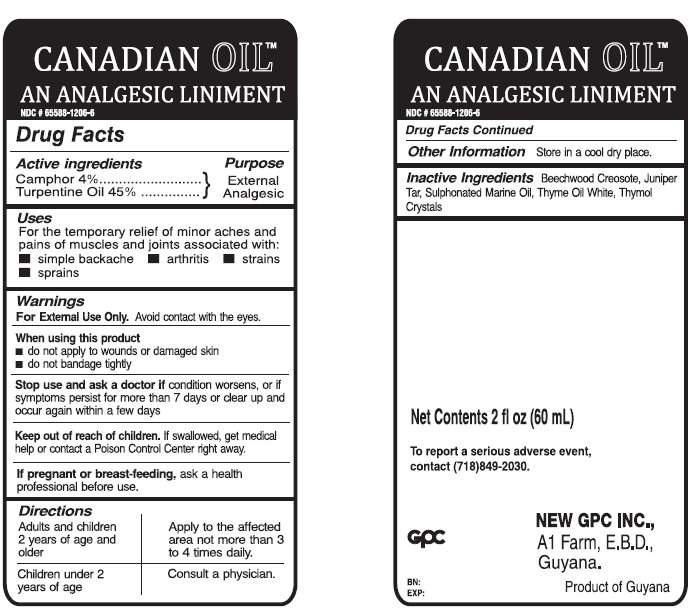
CANADIAN EXTERNAL ANALGESIC
CANADIAN OIL EXTERNAL ANALGESIC LINIMENT
FULL PRESCRIBING INFORMATION: CONTENTS*
- CANADIAN OIL EXTERNAL ANALGESIC LINIMENT
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- WHEN USING THIS PRODUCT
- STOP USE AND ASK A DOCTOR
- IF PREGNANT OR BREAST-FEEDING
- KEEP OUT OF REACH OF CHILDREN.
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- CANADIAN OIL EXTERNAL ANALGESIC LINIMENT 2oz/60ml (65588-1206-6)
FULL PRESCRIBING INFORMATION
CANADIAN OIL EXTERNAL ANALGESIC LINIMENT
ACTIVE INGREDIENTS
Camphor 4%
Turpentine Oil 45%
PURPOSE
External Analgesic
USES
For the temporary relief of minor aches and pains of muscles and joints associated with:
- simple backache
- arthritis
- strains
- sprains
WARNINGS
For External Use Only. Avoid contact with the eyes.
WHEN USING THIS PRODUCT
- do not apply to wounds or damaged skin
- do not bandage tightly
STOP USE AND ASK A DOCTOR
if condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days.
IF PREGNANT OR BREAST-FEEDING
ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN.
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
| Adults and children 2 years of age and older |
Apply to the affected area not more than 3 to 4 times daily |
| Children under 2 years of age |
Consult a physician |
OTHER INFORMATION
Store in a cool dry place.
INACTIVE INGREDIENTS
Beechwood Creosote, Juniper Tar, Sulphonated Marine Oil, Thyme Oil White, Thymol Crystals
CANADIAN OIL EXTERNAL ANALGESIC LINIMENT 2oz/60ml (65588-1206-6)

CANADIAN EXTERNAL ANALGESICCAMPHOR (NATURAL), TURPENTINE OIL LINIMENT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||