CALMS FORTE
CALMS FORTÉ
FULL PRESCRIBING INFORMATION: CONTENTS*
- CALMS FORTE Uses
- Warnings
- Directions
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL - 50 Tablet Bottle Carton
- PRINCIPAL DISPLAY PANEL - 50 Tablet Bottle Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active Ingredients | Purpose |
|---|---|
| Avena Sativa HPUS 1X Double Strength | stress, nervousness |
| Chamomilla HPUS 2X | nervous irritability |
| Humulus Lupulus HPUS 1X Double Strength | occasional sleeplessness |
| Passiflora HPUS 1X Triple Strength | restless sleep from exhaustion |
| Biochemic Phosphates | enhances cellular function |
|
Calc. Phos HPUS 3X, Ferrum Phos HPUS 3X, Kali Phos HPUS 3X, Mag. Phos HPUS 3X, Nat. Phos HPUS 3X |
|
CALMS FORTE Uses
- Temporarily relieves the symptoms of simple nervous tension and occasional sleeplessness.
Warnings
Ask a doctor before use if pregnant or nursing.
Consult a physician if symptoms persist for more than 7 days or worsen.
Keep out of the reach of children.
Do not use if imprinted tamper band is broken or missing.
In case of accidental overdose, contact a poison control center immediately. In case of emergency, Hyland's may be contacted 24 hours a day, 7 days a week at 800/624-9659.
Directions
Adults: As a relaxant: 1 to 2 tablets with water as needed three times daily. For Occasional Sleeplessness: 1 to 3 tablets 1/2 to 1 hour before retiring. Children 6-12 years: 1/2 adult dose.
Inactive Ingredients
Lactose N.F.,Starch (Corn and Tapioca), Vegetable Magnesium Stearate.
PRINCIPAL DISPLAY PANEL - 50 Tablet Bottle Carton
Hyland's
®
HOMEOPATHIC
SINCE 1903
CALMS
FORTÉ
®
SLEEP AID
50
Tablets
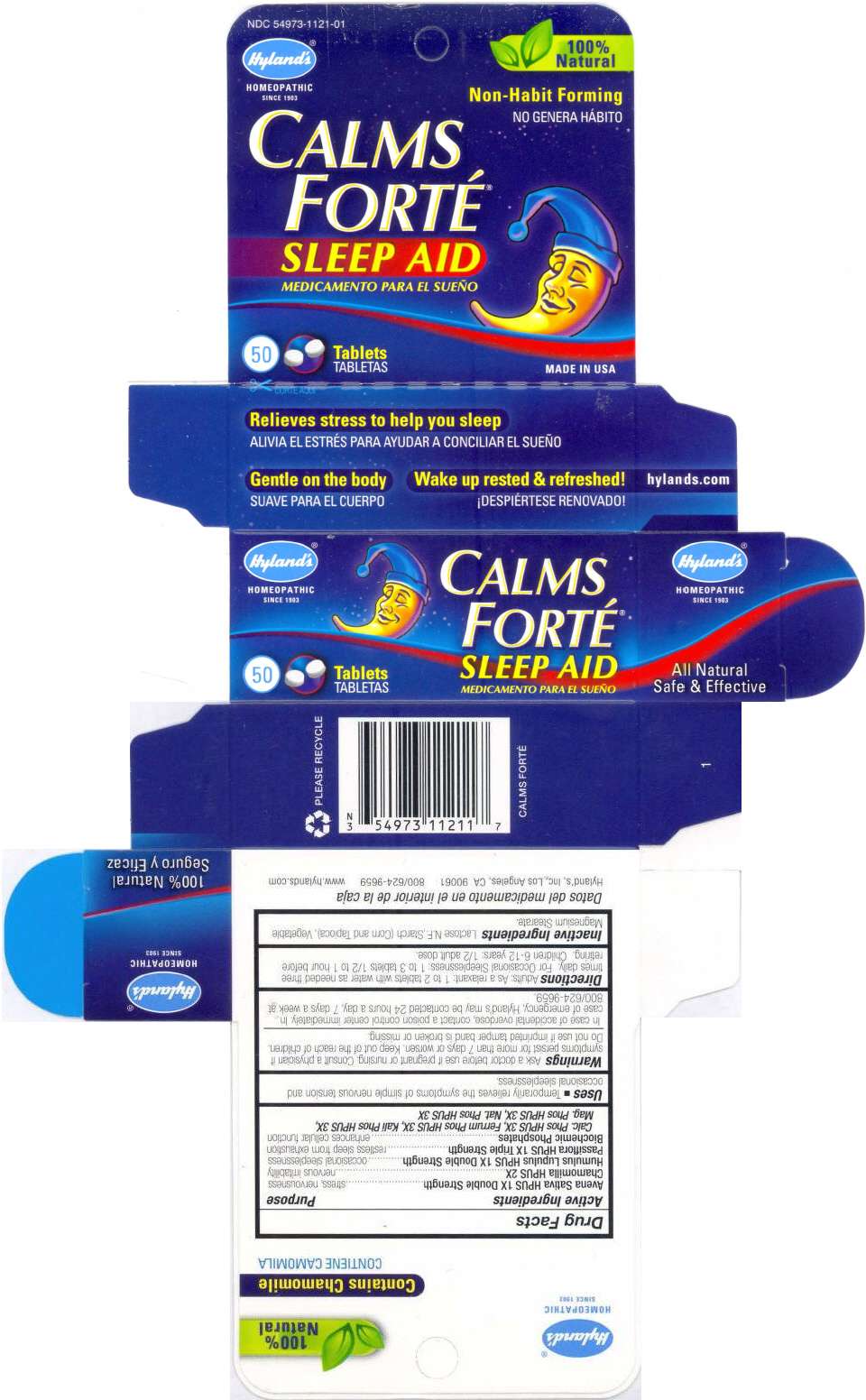
PRINCIPAL DISPLAY PANEL - 50 Tablet Bottle Carton
Hyland's ®
CALMS
FORTÉ
®
SLEEP AID
50
Tablets

CALMS FORTEAVENA SATIVA FLOWERING TOP, CHAMOMILE, HUMULUS LUPULUS STEM, PASSIFLORA INCARNATA FLOWER, CALCIUM PHOSPHATE, FERROSOFERRIC PHOSPHATE, POTASSIUM PHOSPHATE, DIBASIC, SODIUM PHOSPHATE, and MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CALMS FORTEAVENA SATIVA FLOWERING TOP, CHAMOMILE, HUMULUS LUPULUS STEM, PASSIFLORA INCARNATA FLOWER, CALCIUM PHOSPHATE, FERROSOFERRIC PHOSPHATE, POTASSIUM PHOSPHATE, DIBASIC, SODIUM PHOSPHATE, and MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||