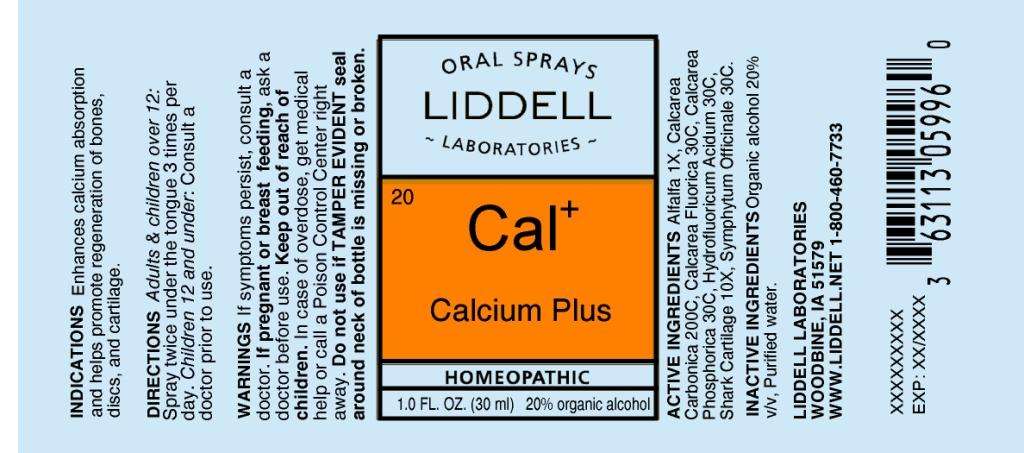
Calcium Plus
Calcium Plus
FULL PRESCRIBING INFORMATION
Active ingredient
ACTIVE INGREDIENTS: Alfalfa 1X, Calcarea carbonica 200C, Calcarea fluorica 30C, Calcarea phosphorica 30C, Hydrofluoricum acidum 30C, Shark cartilage 10X, Symphytum officinale 30C.
Purpose
INDICATIONS: Enhances calcium absorption and helps promote regeneration of bones, discs, and cartilage.
WARNINGS: If symptoms persist, consult a doctor.
If pregnant or breast feeding, ask a doctor before use.
DIRECTIONS: Adults and children over 12: Spray twice under the tongue 3 times per day. Children 12 and under: Consult a doctor prior to use.
INACTIVE INGREDIENTS: Organic alcohol 20% v/v, Purified water.
KEEP OUT OF REACH OF CHILDREN. In case of overdose, get medical help or contact a Poison Control Center right away.
Uses
INDICATIONS: Enhances calcium absorption and helps promote regeneration of bones, discs, and cartilage.
LIDDELL LABORATORIES
WOODBINE, IA 51579
WWW.LIDDELL.NET
1-800-460-7733
ORAL SPRAYS
LIDDELL LABORATORIES
20
Cal
Calcium Plus
HOMEOPATHIC
1.0 FL. OZ. (30 ml)
Calcium PlusAlfalfa, Calcarea carbonica, Calcarea fluorica, Calcarea phosphorica, Hydrofluoricum acidum, Shark cartilage, Symphytum officinale, SPRAY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||