Bupropion Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING
- BUPROPION HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL TRAILS
- INDICATIONS & USAGE
- BUPROPION HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- BUPROPION HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- MEDICATION GUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
WARNING
Suicidality and Antidepressant DrugsUse in Treating Psychiatric Disorders:WARNINGS: Clinical Worsening and Suicide Risk in Treating Psychiatric Disorders,PRECAUTIONS: Information for Patients, andPRECAUTIONS: Pediatric Use).
Use in Smoking Cessation Treatment:Wellbutrinbupropion hydrochloride extended-release tablets (SR), and Wellbutrin XLare not approved for smoking cessation treatment, but bupropion under the name Zybanis approved for this use. Serious neuropsychiatric events, including but not limited to depression, suicidal ideation, suicide attempt, and completed suicide have been reported in patients taking bupropion for smoking cessation. Some cases may have been complicated by the symptoms of nicotine withdrawal in patients who stopped smoking. Depressed mood may be a symptom of nicotine withdrawal. Depression, rarely including suicidal ideation, has been reported in smokers undergoing a smoking cessation attempt without medication. However, some of these symptoms have occurred in patients taking bupropion who continued to smoke.
Advise patients and caregivers that the patient using bupropion for smoking cessation should stop taking bupropion and contact a healthcare provider immediately if agitation, hostility, depressed mood, or changes in thinking or behavior that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior.In many postmarketing cases, resolution of symptoms after discontinuation of Zyban
WARNINGS: Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation TreatmentandPRECAUTIONS: Information for Patients.)
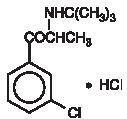
BUPROPION HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Pharmacodynamics:Bupropion is a relatively weak inhibitor of the neuronal uptake of norepinephrine and dopamine, and does not inhibit monoamine oxidase or the re-uptake of serotonin. While the mechanism of action of bupropion, as with other antidepressants, is unknown, it is presumed that this action is mediated by noradrenergic and/or dopaminergic mechanisms.Pharmacokinetics:Bupropion is a racemic mixture. The pharmacologic activity and pharmacokinetics of the individual enantiomers have not been studied. The mean elimination half-life (of bupropion after chronic dosing is 21 (hours, and steady-state plasma concentrations of bupropion are reached within 8 days. In a study comparing chronic dosing with bupropion hydrochloride extended-release tablets (SR) 150 mg twice daily to the immediate-release formulation of bupropion at 100 mg 3 times daily, peak plasma concentrations of bupropion at steady state for bupropion hydrochloride extended-release tablets (SR) were approximately 85% of those achieved with the immediate-release formulation. There was equivalence for bupropion AUCs, as well as equivalence for both peak plasma concentration and AUCs for all 3 of the detectable bupropion metabolites. Thus, at steady state, bupropion hydrochloride extended-release tablets (SR), given twice daily, and the immediate-release formulation of bupropion, given 3 times daily, are essentially bioequivalent for both bupropion and the 3 quantitatively important metabolites.
Absorption:Following oral administration of bupropion hydrochloride extended-release tablets (SR) to healthy volunteers, peak plasma concentrations of bupropion are achieved within 3 hours. Food increased Cmax and AUC of bupropion by 11% and 17%, respectively, indicating that there is no clinically significant food effect.
Distribution:In vitro tests show that bupropion is 84% bound to human plasma proteins at concentrations up to 200 mcg/mL. The extent of protein binding of the hydroxybupropion metabolite is similar to that for bupropion, whereas the extent of protein binding of the threohydrobupropion metabolite is about half that seen with bupropion.
Metabolism:Bupropion is extensively metabolized in humans. Three metabolites have been shown to be active: hydroxybupropion, which is formed via hydroxylation of the tert-butyl group of bupropion, and the amino-alcohol isomers threohydrobupropion and erythrohydrobupropion, which are formed via reduction of the carbonyl group. In vitro findings suggest that cytochrome P450IIB6 (CYP2B6) is the principal isoenzyme involved in the formation of hydroxybupropion, while cytochrome P450 isoenzymes are not involved in the formation of threohydrobupropion. Oxidation of the bupropion side chain results in the formation of a glycine conjugate of metachlorobenzoic acid, which is then excreted as the major urinary metabolite. The potency and toxicity of the metabolites relative to bupropion have not been fully characterized. However, it has been demonstrated in an antidepressant screening test in mice that hydroxybupropion is one half as potent as bupropion, while threohydrobupropion and erythrohydrobupropion are 5-fold less potent than bupropion. This may be of clinical importance because the plasma concentrations of the metabolites are as high or higher than those of bupropion.
PRECAUTIONS: Drug Interactions).
Elimination:Following oral administration of 200 mg of 14C-bupropion in humans, 87% and 10% of the radioactive dose were recovered in the urine and feces, respectively. However, the fraction of the oral dose of bupropion excreted unchanged was only 0.5%, a finding consistent with the extensive metabolism of bupropion.
Population Subgroups:Factors or conditions altering metabolic capacity (e.g., liver disease, congestive heart failure [CHF], age, concomitant medications, etc.) or elimination may be expected to influence the degree and extent of accumulation of the active metabolites of bupropion. The elimination of the major metabolites of bupropion may be affected by reduced renal or hepatic function because they are moderately polar compounds and are likely to undergo further metabolism or conjugation in the liver prior to urinary excretion.
Hepatic:The effect of hepatic impairment on the pharmacokinetics of bupropion was characterized in 2 single-dose studies, one in patients with alcoholic liver disease and one in patients with mild-to-severe cirrhosis. The first study showed that the half-life of hydroxybupropion was significantly longer in 8 patients with alcoholic liver disease than in 8 healthy volunteers (32hours versus 21hours, respectively). Although not statistically significant, the AUCs for bupropion and hydroxybupropion were more variable and tended to be greater (by 53% to 57%) in patients with alcoholic liver disease. The differences in half-life for bupropion and the other metabolites in the 2 patient groups were minimal.
WARNINGS,PRECAUTIONS, andDOSAGE AND ADMINISTRATION).
Renal:There is limited information on the pharmacokinetics of bupropion in patients with renal impairment. An inter-study comparison between normal subjects and patients with end-stage renal failure demonstrated that the parent drug Cmax and AUC values were comparable in the 2 groups, whereas the hydroxybupropion and threohydrobupropion metabolites had a 2.3- and 2.8- fold increase, respectively, in AUC for patients with end-stage renal failure. A second study, comparing normal subjects and patients with moderate-to-severe renal impairment (GFR 30.910.8 mL/min) showed that exposure to a single 150 mg dose of sustained-release bupropion was approximately 2-fold higher in patients with impaired renal function while levels of the hydroxybupropion and threo/erythrohydrobupropion (combined) metabolites were similar in the 2 groups. The elimination of bupropion and/or the major metabolites of bupropion may be reduced by impaired renal function (seePRECAUTIONS: Renal Impairment).
Left Ventricular Dysfunction:During a chronic dosing study with bupropion in 14 depressed patients with left ventricular dysfunction (history of CHF or an enlarged heart on x-ray), no apparent effect on the pharmacokinetics of bupropion or its metabolites was revealed, compared to healthy volunteers.
Age:PRECAUTIONS: Geriatric Use).
Gender:A single-dose study involving 12 healthy male and 12 healthy female volunteers revealed no sex-related differences in the pharmacokinetic parameters of bupropion.
Smokers:
CLINICAL TRAILS
INDICATIONS & USAGE
CLINICAL PHARMACOLOGY).
A major depressive episode (DSM-IV) implies the presence of 1)depressed mood or 2) loss of interest or pleasure; in addition, at least 5 of the following symptoms have been present during the sa,e 2-week period and represent a change from previous functioning: depressed mood, markedly diminished interest or pleasure in usual activities, significant change in weight and/orappetite, insomina or hypersomnia, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, a suicide attempt or suicidal ideation.
CLINICAL PHARMACOLOGY). Nevertheless, the physician who elects to use bupropion hydrochloride extended-release tablets (SR) for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient.
BUPROPION HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Clinical Worsening and Suicide Risk in Treating Psychiatric Disorders:Patients with major depressive disorder (MDD), both adult and pediatric, amy experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults ages 65 and older.
Table 1
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers.Prescriptions for bupropion hydrochloride extended-release tablets (SR) should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation Treatment:Wellbutrinbupropion hydrochloride extended-release tablets (SR), and Wellbutrin XLnot approved for smoking cessation treatment, but bupropion under the name Zybanis approved for this use. Serious neuropsychiatric symptoms have been reported in patients taking bupropion for smoking cessation (see BOXED WARNING, ADVERSE REACTIONS). These have included changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, hostility, agitation, aggression, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide.Some reported cases may have been complicated by the symptoms of nicotine withdrawal in patients who stopped smoking. Depressed mood may be a symptom of nicotine withdrawal. Depression, rarely including suicidal ideation, has been reported in smokers undergoing a smoking cessation attempt without medication. However, some of these symptoms have occurred in patients taking bupropion who continued to smoke. When symptoms were reported, most were during bupropion treatment, but some were following discontinuation of bupropion therapy.
Advise patients and caregivers that the patient using bupropion for smoking cessation should stop taking bupropion and contact a healthcare provider immediately if agitation, depressed mood, or changes in behavior or thinking that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior. In many postmarketing cases, resolution of symptoms after discontinuation of Zybanwas reported, although in some cases the symptoms persisted, therefore, ongoing monitoring and supportive care should be provided until symptoms resolve.
Screening Patients for Bipolar Disorder:
Bupropion-Containing Products:Patients should be made aware that bupropion hydrochloride extended-release tablets (SR) contain the same active ingredient found in Zybanused as an aid to smoking cessation treatment, and that bupropion hydrochloride extended-release tablets should not be used in combination with Zybanor any other medications that contain bupropion, such as Wellbutrin(bupropion hydrochloride), the immediate-release formulation or Wellbutrin XL(bupropion hydrochloride), the extended-release formulation.
Seizures: Bupropion is associated with a dose-related risk of seizures. The risk of seizures is also related to patient factors, clinical situations, and concomitant medications, which must be considered in selection of patients for therapy with bupropion hydrochloride extendedrelease tablets (SR). Bupropion hydrochloride extended-release tablets (SR) should be discontinued and not restarted in patients who experience a seizure while on treatment.
Dose: At doses of sustained-release bupropion up to a dose of 300 mg/day, the incidence of seizure is approximately 0.1% (1/1,000) and increases to approximately 0.4% (4/1,000) at the maximum recommended dose of 400 mg/day.
Data for the immediate-release formulation of bupropion revealed a seizure incidence of approximately 0.4% (i.e., 13 of 3,200 patients followed prospectively) in patients treated at doses in a range of 300 to 450 mg/day. The 450 mg/day upper limit of this dose range is close to the currently recommended maximum dose of 400 mg/day for bupropion hydrochloride extended-release tablets (SR). This seizure incidence (0.4%) may exceed that of other marketed antidepressants and bupropion hydrochloride extended-release tablets (SR) up to 300 mg/day by as much as 4-fold. This relative risk is only an approximate estimate because no direct comparative studies have been conducted.
Additional data accumulated for the immediate-release formulation of bupropion suggested that the estimated seizure incidence increases almost tenfold between 450 and 600 mg/day, which is twice the usual adult dose and one and one-half the maximum recommended daily dose (400 mg) of bupropion hydrochloride extended-release tablets (SR). This disproportionate increase in seizure incidence with dose incrementation calls for caution in dosing.
Data for bupropion hydrochloride extended-release tablets (SR) revealed a seizure incidence of approximately 0.1% (i.e., 3 of 3,100 patients followed prospectively) in patients treated at doses in a range of 100 to 300 mg/day. It is not possible to know if the lower seizure incidence observed in this study involving the sustained-release formulation of bupropion resulted from the different formulation or the lower dose used. However, as noted above, the immediate-release and sustained-release formulations are bioequivalent with regard to both rate and extent of absorption during steady state (the most pertinent condition to estimating seizure incidence), since most observed seizures occur under steady-state conditions.
Patient factors: Predisposing factors that may increase the risk of seizure with bupropion use include history of head trauma or prior seizure, central nervous system (CNS) tumor, the presence of severe hepatic cirrhosis, and concomitant medications that lower seizure threshold.
Concomitant medications: Many medications (e.g., antipsychotics, antidepressants, theophylline, systemic steroids) are known to lower seizure threshold.
Recommendations for Reducing the Risk of Seizure: Retrospective analysis of clinical experience gained during the development of bupropion suggests that the risk of seizure may be minimized if
the total daily dose of bupropion hydrochloride extended-release tablets (SR) does not exceed 400 mg,
the daily dose is administered twice daily, and
the rate of incrementation of dose is gradual.
No single dose should exceed 200 mg to avoid high peak concentrations of bupropion and/or its metabolites.
Bupropion hydrochloride extended-release tablets (SR) should be administered with extreme caution to patients with a history of seizure, cranial trauma, or other predisposition(s) toward seizure, or patients treated with other agents (e.g., antipsychotics, other antidepressants, theophylline, systemic steroids, etc.) that lower seizure threshold.
Hepatic Impairment: Bupropion hydrochloride extended-release tablets (SR) should be used with extreme caution in patients with severe hepatic cirrhosis. In these patients a reduced frequency and/or dose is required, as peak bupropion, as well as AUC, levels are substantially increased and accumulation is likely to occur in such patients to a greater extent than usual. The dose should not exceed 100 mg every day or 150 mg every other day in these patients (see CLINICAL PHARMACOLOGY, PRECAUTIONS, and DOSAGE AND ADMINISTRATION).
Potential for Hepatotoxicity:In rats receiving large doses of bupropion chronically, there was an increase in incidence of hepatic hyperplastic nodules and hepatocellular hypertrophy. In dogs receiving large doses of bupropion chronically, various histologic changes were seen in the liver, and laboratory tests suggesting mild hepatocellular injury were noted.
PRECAUTIONS
General:Agitation and Insomnia:Patients in placebo-controlled trials with bupropion hydrochloride extended-release tablets (SR) experienced agitation, anxiety, and insomnia as shown in Table 2.
Table 2. Incidence of Agitation, Anxiety, and Insomnia in Placebo-Controlled Trials
Psychosis, Confusion, and Other Neuropsychiatric Phenomena:Depressed patients treated with an immediate-release formulation of bupropion or with bupropion hydrochloride extended-release tablets (SR) have been reported to show a variety of neuropsychiatric signs and symptoms, including delusions, hallucinations, psychosis, concentration disturbance, paranoia, and confusion. In some cases, these symptoms abated upon dose reduction and/or withdrawal of treatment.
Activation of Psychosis and/or Mania:Antidepressants can precipitate manic episodes in bipolar disorder patients during the depressed phase of their illness and may activate latent psychosis in other susceptible patients. The sustained-release formulation of bupropion is expected to pose similar risks.
Altered Appetite and Weight:In placebo-controlled studies, patients experienced weight gain or weight loss as shown in Table 3.
Table 3. Incidence of Weight Gain and Weight Loss in Placebo-Controlled Trials
Allergic Reactions:Anaphylactoid/anaphylactic reactions characterized by symptoms such as pruritus, urticaria, angioedema, and dyspnea requiring medical treatment have been reported in clinical trials with bupropion. In addition, there have been rare spontaneous postmarketing reports of erythema multiforme, Stevens-Johnson syndrome, and anaphylactic shock associated with bupropion. A patient should stop taking bupropion hydrochloride extended-release tablets (SR) and consult a doctor if experiencing allergic or anaphylactoid/anaphylactic reactions (e.g., skin rash, pruritus, hives, chest pain, edema, and shortness of breath) during treatment.
Cardiovascular Effects:
Hepatic Impairment:Bupropion hydrochloride extended-release tablets (SR) should be used with extreme caution in patients with severe hepatic cirrhosis. In these patients, a reduced frequency and/or dose is required. Bupropion hydrochloride extended-release tablets (SR) should be used with caution in patients with hepatic impairment (including mild-to-moderate hepatic cirrhosis) and reduced frequency and/or dose should be considered in patients with mild-to-moderate hepatic cirrhosis.
CLINICAL PHARMACOLOGY,WARNINGS, andDOSAGE AND ADMINISTRATION).
Renal Impairment:
INFORMATION FOR PATIENTS
Clinical Worsening and Suicide Risk in Treating Psychiatric Disorders:Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patientprescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patientpresenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation Treatment:Although bupropion hydrochloride extended-release tablets (SR) are not indicated for smoking cessation treatment, it contains the same active ingredient as Zybanwhich is approved for this use. Patients should be informed that quitting smoking, with or without Zybanmay be associated with nicotine withdrawal symptoms (including depression or agitation), or exacerbation of pre-existing psychiatric illness. Furthermore, some patients have experienced changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, aggression, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide when attempting to quit smoking while taking ZybanIf patients develop agitation, hostility, depressed mood, or changes in thinking or behavior that are not typical for them, or if patients develop suicidal ideation or behavior, they should be urged to report these symptoms to their healthcare provider immediately.
Bupropion-Containing Products:Patients should be made aware that bupropion hydrochloride extended-release tablets (SR) contain the same active ingredient found in Zybanused as an aid to smoking cessation treatment, and that bupropion hydrochloride extended-release tablets should not be used in combination with Zybanor any other medications that contain bupropion hydrochloride (such as Wellbutrinthe immediate-release formulation and Wellbutrin XLthe extended-release formulation).
LABORATORY TESTS
DRUG INTERACTIONS
Drugs Metabolized By Cytochrome P450IID6 (CYP2D6):Many drugs, including most antidepressants (SSRIs, many tricyclics), beta-blockers, antiarrhythmics, and antipsychotics are metabolized by the CYP2D6 isoenzyme. Although bupropion is not metabolized by this isoenzyme, bupropion and hydroxybupropion are inhibitors of CYP2D6 isoenzyme in vitro. In a study of 15 male subjects (ages 19 to 35 years) who were extensive metabolizers of the CYP2D6 isoenzyme, daily doses of bupropion given as 150 mg twice daily followed by a single dose of 50 mg desipramine increased the Cmax, AUC, and tof desipramine by an average of approximately 2-, 5-, and 2-fold, respectively. The effect was present for at least 7 days after the last dose of bupropion. Concomitant use of bupropion with other drugs metabolized by CYP2D6 has not been formally studied.
MAO Inhibitors:Studies in animals demonstrate that the acute toxicity of bupropion is enhanced by the MAO inhibitor phenelzine (seeCONTRAINDICATIONS).
Levodopa and Amantadine:Limited clinical data suggest a higher incidence of adverse experiences in patients receiving bupropion concurrently with either levodopa or amantadine. Administration of bupropion hydrochloride extended-release tablets (SR) to patients receiving either levodopa or amantadine concurrently should be undertaken with caution, using small initial doses and gradual dose increases.
Drugs That Lower Seizure Threshold:Concurrent administration of bupropion hydrochloride extended-release tablets (SR) and agents (e.g., antipsychotics, other antidepressants, theophylline, systemic steroids, etc.) that lower seizure threshold should be undertaken only with extreme caution (seeWARNINGS). Low initial dosing and gradual dose increases should be employed.
Nicotine Transdermal System:(seePRECAUTIONS: Cardiovascular Effects).
Alcohol:In postmarketing experience, there have been rare reports of adverse neuropsychiatric events or reduced alcohol tolerance in patients who were drinking alcohol during treatment with bupropion extended-release tablets (SR). The consumption of alcohol during treatment with bupropion extended-release tablets (SR) should be minimized or avoided (also seeCONTRAINDICATIONS).
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
BOX WARNINGandWARNINGS: Clinical Worsening and Suicide Risk in Treating Psychiatric Disorders). Anyone considering the use of bupropion in a child or adolescent must balance the potential risks with the clinical need.GERIATRIC USE
CLINICAL PHARMACOLOGY).
PRECAUTIONS: Renal ImpairmentandDOSAGE AND ADMINISTRATION).
BUPROPION HYDROCHLORIDE ADVERSE REACTIONS
WARNINGSandPRECAUTIONS.)Incidence in Controlled Trials With Bupropion:
Adverse Events Associated With Discontinuation of Treatment Among Patients Treated With Bupropion Hydrochloride Extended-Release Tablets (SR):In placebo-controlled clinical trials, 9% and 11% of patients treated with 300 and 400 mg/day, respectively, of bupropion hydrochloride extended-release tablets (SR) and 4% of patients treated with placebo discontinued treatment due to adverse events. The specific adverse events in these trials that led to discontinuation in at least 1% of patients treated with either 300 mg/day or 400 mg/day of bupropion hydrochloride extended-release tablets (SR) and at a rate at least twice the placebo rate are listed in Table 4.
Table 4. Treatment Discontinuations Due to Adverse Events in Placebo-Controlled Trials
Adverse Events Occurring at an Incidence of 1% or More Among Patients Treated With Bupropion Hydrochloride Extended-Release Tablets (SR):Table 5 enumerates treatment-emergent adverse events that occurred among patients treated with 300 and 400 mg/day of bupropion hydrochloride extended-release tablets (SR) and with placebo in placebo-controlled trials. Events that occurred in either the 300- or 400-mg/day group at an incidence of 1% or more and were more frequent than in the placebo group are included. Reported adverse events were classified using a COSTART-based Dictionary.
Table 5. Treatment-Emergent Adverse Events in Placebo-Controlled Trials*
Incidence of Commonly Observed Adverse Events in Controlled Clinical Trials:Adverse events from Table 5 occurring in at least 5% of patients treated with bupropion hydrochloride extended release tablets (SR) and at a rate at least twice the placebo rate are listed below for the 300- and 400-mg/day dose groups.
Bupropion Extended-release Tablets (SR) 300 mg/day:Anorexia, dry mouth, rash, sweating, tinnitus, and tremor.
Bupropion Extended-release Tablets (SR) 400 mg/day:Abdominal pain, agitation, anxiety, dizziness, dry mouth, insomnia, myalgia, nausea, palpitation, pharyngitis, sweating, tinnitus, and urinary frequency.
Other Events Observed During the Clinical Development and Postmarketing Experience of Bupropion:
Body (General):Infrequent were chills, facial edema, musculoskeletal chest pain, and photosensitivity. Rare was malaise. Also observed were arthralgia, myalgia, and fever with rash and other symptoms suggestive of delayed hypersensitivity. These symptoms may resemble serum sickness (seePRECAUTIONS).
Cardiovascular:Infrequent were postural hypotension, stroke, tachycardia, and vasodilation. Rare was syncope. Also observed were complete atrioventricular block, extrasystoles, hypotension, hypertension (in some cases severe, seePRECAUTIONS), myocardial infarction, phlebitis, and pulmonary embolism.
Digestive:Infrequent were abnormal liver function, bruxism, gastric reflux, gingivitis, glossitis, increased salivation, jaundice, mouth ulcers, stomatitis, and thirst. Rare was edema of tongue. Also observed were colitis, esophagitis, gastrointestinal hemorrhage, gum hemorrhage, hepatitis, intestinal perforation, liver damage, pancreatitis, and stomach ulcer.
Endocrine:Also observed were hyperglycemia, hypoglycemia, and syndrome of inappropriate antidiuretic hormone.
Hemic and Lymphatic:Infrequent was ecchymosis. Also observed were anemia, leukocytosis, leukopenia, lymphadenopathy, pancytopenia, and thrombocytopenia. Altered PT and/or INR, infrequently associated with hemorrhagic or thrombotic complications, were observed when bupropion was coadministered with warfarin.
Metabolic and Nutritional:Infrequent were edema and peripheral edema. Also observed was glycosuria.
Musculoskeletal:Infrequent were leg cramps. Also observed were muscle rigidity/fever/rhabdomyolysis and muscle weakness.
Nervous System:Infrequent were abnormal coordination, decreased libido, depersonalization, dysphoria, emotional lability, hostility, hyperkinesia, hypertonia, hypesthesia, suicidal ideation, and vertigo. Rare were amnesia, ataxia, derealization, and hypomania. Also observed were abnormal electroencephalogram (EEG), akinesia, aggression, aphasia, coma, completed suicide, delirium, delusions, dysarthria, dyskinesia, dystonia, euphoria, extrapyramidal syndrome, hallucinations, hypokinesia, increased libido, manic reaction, neuralgia, neuropathy, paranoid ideation, restlessness, suicide attempt, and unmasking tardive dyskinesia.
Respiratory:Rare was bronchospasm. Also observed was pneumonia.
Skin:Rare was maculopapular rash. Also observed were alopecia, angioedema, exfoliative dermatitis, and hirsutism.
Special Senses:Infrequent were accommodation abnormality and dry eye. Also observed were deafness, diplopia, increased intraocular pressure, and mydriasis.
Urogenital:Infrequent were impotence, polyuria, and prostate disorder. Also observed were abnormal ejaculation, cystitis, dyspareunia, dysuria, gynecomastia, menopause, painful erection, salpingitis, urinary incontinence, urinary retention, and vaginitis.
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class:Bupropion is not a controlled substance.Humans:Controlled clinical studies of bupropion (immediate-release formulation) conducted in normal volunteers, in subjects with a history of multiple drug abuse, and in depressed patients showed some increase in motor activity and agitation/excitement.
Animals:Studies in rodents and primates have shown that bupropion exhibits some pharmacologic actions common to psychostimulants. In rodents, it has been shown to increase locomotor activity, elicit a mild stereotyped behavioral response, and increase rates of responding in several schedule-controlled behavior paradigms. In primate models to assess the positive reinforcing effects of psychoactive drugs, bupropion was self-administered intravenously. In rats, bupropion produced amphetamine-like and cocaine-like discriminative stimulus effects in drug discrimination paradigms used to characterize the subjective effects of psychoactive drugs.
OVERDOSAGE
Human Overdose Experience:Overdoses of up to 30 g or more of bupropion have been reported. Seizure was reported in approximately one-third of all cases. Other serious reactions reported with overdoses of bupropion alone included hallucinations, loss of consciousness, sinus tachycardia, and ECG changes such as conduction disturbances (including QRS prolongation) or arrhythmias. Fever, muscle rigidity, rhabdomyolysis, hypotension, stupor, coma, and respiratory failure have been reported mainly when bupropion was part of multiple drug overdoses.Overdosage Management:Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. EEG monitoring is also recommended for the first 48 hours post-ingestion. General supportive and symptomatic measures are also recommended. Induction of emesis is not recommended.
DOSAGE & ADMINISTRATION
General Dosing Considerations:It is particularly important to administer bupropion hydrochloride extended-release tablets (SR) in a manner most likely to minimize the risk of seizure (seeWARNINGS). Gradual escalation in dosage is also important if agitation, motor restlessness, and insomnia, often seen during the initial days of treatment, are to be minimized. If necessary, these effects may be managed by temporary reduction of dose or the short-term administration of an intermediate to long-acting sedative hypnotic. A sedative hypnotic usually is not required beyond the first week of treatment. Insomnia may also be minimized by avoiding bedtime doses. If distressing, untoward effects supervene, dose escalation should be stopped. Bupropion hydrochloride extended-release tablets (SR) should be swallowed whole and not crushed, divided, or chewed, as this may lead to increased risk of adverse effects including seizures.Initial Treatment:The usual adult target dose for bupropion hydrochloride extended-release tablets (SR) is 300 mg/day, given as 150 mg twice daily. Dosing with bupropion hydrochloride extended-release tablets (SR) should begin at 150 mg/day given as a single daily dose in the morning. If the 150 mg initial dose is adequately tolerated, an increase to the 300 mg/day target dose, given as 150 mg twice daily, may be made as early as day 4 of dosing. There should be an interval of at least 8 hours between successive doses.
Increasing the Dosage Above 300 mg/day:As with other antidepressants, the full antidepressant effect of bupropion hydrochloride extended-release tablets (SR) may not be evident until 4 weeks of treatment or longer. An increase in dosage to the maximum of 400 mg/day, given as 200 mg twice daily, may be considered for patients in whom no clinical improvement is noted after several weeks of treatment at 300 mg/day.
Maintenance Treatment:It is generally agreed that acute episodes of depression require several months or longer of sustained pharmacological therapy beyond response to the acute episode. In a study in which patients with major depressive disorder, recurrent type, who had responded during 8 weeks of acute treatment with bupropion hydrochloride extended-release tablets (SR) were assigned randomly to placebo or to the same dose of bupropion hydrochloride extended-release tablets (SR) (150 mg twice daily) during 44 weeks of maintenance treatment as they had received during the acute stabilization phase, longer-term efficacy was demonstrated (seeCLINICAL TRIALSunderCLINICAL PHARMACOLOGY). Based on these limited data, it is unknown whether or not the dose of bupropion hydrochloride extended-release tablets (SR) needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment and the appropriate dose for such treatment.
Dosage Adjustment for Patients with Impaired Hepatic Function:Bupropion hydrochloride extended-release tablets (SR) should be used with extreme caution in patients with severe hepatic cirrhosis. The dose should not exceed 100 mg every day or 150 mg every other day in these patients. Bupropion hydrochloride extended-release tablets (SR) should be used with caution in patients with hepatic impairment (including mild-to-moderate hepatic cirrhosis) and a reduced frequency and/or dose should be considered in patients with mild-to-moderate hepatic cirrhosis (seeCLINICAL PHARMACOLOGY,WARNINGS, andPRECAUTIONS).
Dosage Adjustment for Patients with Impaired Renal Function:Bupropion hydrochloride extended-release tablets (SR) should be used with caution in patients with renal impairment and a reduced frequency and/or dose should be considered (seeCLINICAL PHARMACOLOGYandPRECAUTIONS).
HOW SUPPLIED
WPIover858in bottles of 60 tablets (NDC 0591-3540-60), 100 tablets (NDC 0591-3540-01) and 500 tablets (NDC 0591-3540-05).WPIover839in bottles of 60 tablets (NDC 0591-3541-60), 100 tablets (NDC 0591-3541-01), 250 tablets (NDC 0591-3541-25) and 500 tablets (NDC 0591-3541-05).
WPIover3385in bottles of 60 tablets (NDC 0591-3542-60), 100 tablets (NDC 0591-3542-01) and 500 tablets (NDC 0591-3542-05).
STORAGE AND HANDLING
Store at 20to 25(68to 77[See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP.MEDICATION GUIDE
BuPROPion Hydrochloride Extended-release Tablets USP (SR)other important information should I know about bupropion hydrochloride extended-release tablets (SR)?
Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Talk to your, or your family memberhealthcare provider about:
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
What else do I need to know about antidepressant medicines?
Never stop an antidepressant medicine without first talking to a healthcare provider.Stopping an antidepressant medicine suddenly can cause other symptoms.
Antidepressants are medicines used to treat depression and other illnesses.It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
Antidepressant medicines have other side effects.Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
Antidepressant medicines can interact with other medicines.Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
Not all antidepressant medicines prescribed for children are FDA approved for use in children.Talk to your childhealthcare provider for more information.
Quitting Smoking, Quit-Smoking Medications, Changes in Thinking and Behavior, Depression, and Suicidal Thoughts or Actions
What Other Important Information Should I Know About Bupropion Hydrochloride Extended-release Tablets (SR)?
Seizures: There is a chance of having a seizure (convulsion, fit) with bupropion hydrochloride extended-release tablets (SR), especially in people:
Do not take any other medicines while you are using bupropion hydrochloride extended-release tablets (SR) unless your doctor has said it is okay to take them.
f you have a seizure while taking bupropion hydrochloride extended-release tablets (SR), stop taking the tablets and call your doctor right away.Do not take bupropion hydrochloride extended-release tablets (SR) again if you have a seizure.
High blood pressure (hypertension). Some people get high blood pressure, that can be severe, while taking bupropion hydrochloride extended-release tablets (SR).The chance of high blood pressure may be higher if you also use nicotine replacement therapy (such as a nicotine patch) to help you stop smoking.
Severe allergic reactions. Some people have severe allergic reaction to bupropion hydrochloride extended-release tablets (SR). Stop taking bupropion hydrochloride extended-release tablets (SR) and call your doctor right awayif you get a rash, itching, hives, fever, swollen lymph glands, painful sores in the mouth or around the eyes, swelling of the lips or tongue, chest pain, or have trouble breathing. These could be signs of a serious allergic reaction.
Unusual thoughts or behaviors.Some patients have unusual thoughts or behaviors while taking bupropion hydrochloride extended-release tablets (SR), including delusions (believe you are someone else), hallucinations (seeing or hearing things that are not there), paranoia (feeling that people are against you), or feeling confused. If this happens to you, call your doctor.
What are bupropion hydrochloride extended-release tablets (SR)?
Who should not take bupropion hydrochloride extended-release tablets (SR)?
Do not take bupropion hydrochloride extended-release tablets (SR) if you:
are taking Zyban(used to help people stop smoking) or any other medicines that contain bupropion hydrochloride, such as WellbutrinTablets or Wellbutrin XLExtended-Release Tablets.Bupropion is the same active ingredient that is in bupropion hydrochloride extended-release tablets (SR).
What should I tell my doctor before using bupropion hydrochloride extended-release tablets (SR)?
Tell your doctor about your other medical conditions including if you:
are pregnant or plan to become pregnant.It is not known if bupropion hydrochloride extended-release tablets (SR) can harm your unborn baby.
are breastfeeding.Bupropion passes through your milk. It is not known if bupropion can harm your baby.
have liver problems, especially cirrhosis of the liver.
Tell your doctor about all the medicines you take, including prescription and non- prescription medicines, vitamins, and herbal supplements. Many medicines increase your chances of having seizures or other serious side effects if you take them while you are using bupropion hydrochloride extended-release tablets (SR).
How should I take bupropion hydrochloride extended-release tablets (SR)?
Do not chew, cut, or crush bupropion hydrochloride extended-release tablets (SR).If you do, the medicine will be released into your body too quickly. If this happens you may be more likely to get side effects including seizures. You must swallow the tablets whole.Tell your doctor if you cannot swallow medicine tablets.
This is very important.Too many bupropion hydrochloride extended-release tablets (SR) can increase your chance of having a seizure.
Do not take any other medicines while using bupropion hydrochloride extended-release tablets (SR) unless your doctor has told you it is okay.
What should I avoid while taking bupropion hydrochloride extended-release tablets (SR)?
What are possible side effects of bupropion hydrochloride extended-release tablets (SR)?
How should I store bupropion hydrochloride extended-release tablets (SR)?
General Information about bupropion hydrochloride extended-release tablets (SR).
What are the ingredients in bupropion hydrochloride extended-release tablets (SR)?
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Bupropion HydrochlorideBUPROPION HYDROCHLORIDE TABLET, FILM COATED, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!