Bupropion hydrochloride
Bupropion Hydrochloride Extended-Release Tablets, USP (SR)
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING
- BUPROPION HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- BUPROPION HYDROCHLORIDE INDICATIONS AND USAGE
- BUPROPION HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- BUPROPION HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- BUPROPION HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- MEDICATION GUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 100MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 150MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 200MG
FULL PRESCRIBING INFORMATION
WARNING
Suicidality and Antidepressant Drugs
Use in Treating Psychiatric Disorders: WARNINGS: Clinical Worsening and Suicide Risk in Treating Psychiatric DisordersPRECAUTIONS: Information for PatientsPRECAUTIONS: Pediatric Use
Use in Smoking Cessation Treatment: ®#
####
Advise patients and caregivers that the patient using bupropion for smoking cessation should stop taking bupropion and contact a healthcare provider immediately if agitation, hostility, depressed mood, or changes in thinking or behavior that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior. In many postmarketing cases, resolution of symptoms after discontinuation of ZYBAN# was reported, although in some cases the symptoms persisted; therefore, ongoing monitoring and supportive care should be provided until symptoms resolve.
#WARNINGS: Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation TreatmentPRECAUTIONS: Information for Patients
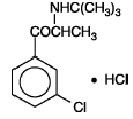
BUPROPION HYDROCHLORIDE DESCRIPTION
1318
CLINICAL PHARMACOLOGY
Pharmacodynamics:
Pharmacokinetics:
Absorption: max
Distribution:
Metabolism: tert
PRECAUTIONS: Drug Interactions
Elimination: 14
Population Subgroups:
Hepatic:
maxmax½maxmaxmax maxWARNINGSPRECAUTIONSDOSAGE AND ADMINISTRATION
Renal: max PRECAUTIONS: Renal Impairment
Left Ventricular Dysfunction:
Age: PRECAUTIONS: Geriatric Use
Gender:
Smokers: maxmax
CLINICAL TRIALS
BUPROPION HYDROCHLORIDE INDICATIONS AND USAGE
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
BUPROPION HYDROCHLORIDE CONTRAINDICATIONS
#
WARNINGS
Clinical Worsening and Suicide Risk in Treating Psychiatric Disorders:
| Age Range |
Drug-Placebo Difference in Number of Cases of Suicidality per 1,000 Patients Treated |
| Increases Compared to Placebo |
|
| <18 |
14 additional cases |
| 18 to 24 |
5 additional cases |
| Decreases Compared to Placebo |
|
| 25 to 64 |
1 fewer case |
| ≥65 |
6 fewer cases |
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.
Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation Treatment:
#(see BOXED WARNING, ADVERSE REACTIONS). These have included changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, hostility, agitation, aggression, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide.
#
Advise patients and caregivers that the patient using bupropion for smoking cessation should stop taking bupropion and contact a healthcare provider immediately if agitation, depressed mood, or changes in behavior or thinking that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior. In many postmarketing cases, resolution of symptoms after discontinuation of ZYBAN# was reported, although in some cases the symptoms persisted, therefore, ongoing monitoring and supportive care should be provided until symptoms resolve.
#
Screening Patients for Bipolar Disorder:
Bupropion-Containing Products
®#®#
Seizures:
Bupropion is associated with a dose-related risk of seizures. The risk of seizures is also related to patient factors, clinical situations, and concomitant medications, which must be considered in selection of patients for therapy with bupropion. Bupropion should be discontinued and not restarted in patients who experience a seizure while on treatment.
- Dose: At doses of bupropion up to a dose of 300 mg/day, the incidence of seizure is approximately 0.1% (1/1,000) and increases to approximately 0.4% (4/1,000) at the maximum recommended dose of 400 mg/day.
Additional data accumulated for the immediate-release formulation of bupropion suggested that the estimated seizure incidence increases almost tenfold between 450 and 600 mg/day, which is twice the usual adult dose and one and one-half the maximum recommended daily dose (400 mg) of bupropion hydrochloride extended-release tablets (SR). This disproportionate increase in seizure incidence with dose incrementation calls for caution in dosing.
Data for bupropion hydrochloride extended-release tablets (SR) revealed a seizure incidence of approximately 0.1% (i.e., 3 of 3,100 patients followed prospectively) in patients treated at doses in a range of 100 to 300 mg/day. It is not possible to know if the lower seizure incidence observed in this study involving the sustained-release formulation of bupropion resulted from the different formulation or the lower dose used. However, as noted above, the immediate-release and sustained-release formulations are bioequivalent with regard to both rate and extent of absorption during steady state (the most pertinent condition to estimating seizure incidence), since most observed seizures occur under steady-state conditions.
- Patient factors: Predisposing factors that may increase the risk of seizure with bupropion use include history of head trauma or prior seizure, central nervous system (CNS) tumor, the presence of severe hepatic cirrhosis, and concomitant medications that lower seizure threshold.
- Clinical situations: Circumstances associated with an increased seizure risk include, among others, excessive use of alcohol or sedatives (including benzodiazepines); addiction to opiates, cocaine, or stimulants; use of over-the-counter stimulants and anorectics; and diabetes treated with oral hypoglycemics or insulin.
- Concomitant medications: Many medications (e.g., antipsychotics, antidepressants, theophylline, systemic steroids) are known to lower seizure threshold.
- the total daily dose of bupropion hydrochloride extended-release tablets (SR) does not exceed 400 mg,
- the daily dose is administered twice daily, and
- the rate of incrementation of dose is gradual.
- No single dose should exceed 200 mg to avoid high peak concentrations of bupropion and/or its metabolites.
Hepatic Impairment:
Bupropion should be used with extreme caution in patients with severe hepatic cirrhosis. In these patients a reduced frequency and/or dose is required, as peak bupropion, as well as AUC, levels are substantially increased and accumulation is likely to occur in such patients to a greater extent than usual. The dose should not exceed 100 mg every day or 150 mg every other day in these patients (see CLINICAL PHARMACOLOGY, PRECAUTIONS, and DOSAGE AND ADMINISTRATION).
Potential for Hepatotoxicity:
PRECAUTIONS
General
Agitation and Insomnia:
| Adverse Event Term |
Bupropion 300 mg/day (n = 376) |
Bupropion 400 mg/day (n = 114) |
Placebo (n = 385) |
| Agitation Anxiety Insomnia |
3% 5% 11% |
9% 6% 16% |
2% 3% 6% |
Psychosis, Confusion, and Other Neuropsychiatric Phenomena:
Activation of Psychosis and/or Mania:
Altered Appetite and Weight:
In placebo-controlled studies, patients experienced weight gain or weight loss as shown in Table 3.
| Weight Change |
Bupropion 300 mg/day (n = 339) |
Bupropion 400 mg/day (n = 112) |
Placebo (n = 347) |
| Gained >5 lbs Lost >5 lbs |
3% 14% |
2% 19% |
4% 6% |
Allergic Reactions:
Cardiovascular Effects:
®###
Hepatic Impairment:
CLINICAL PHARMACOLOGYWARNINGSDOSAGE AND ADMINISTRATION
Renal Impairment:
max
Information for Patients
Clinical Worsening and Suicide Risk in Treating Psychiatric Disorders:
Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation Treatment: ###
Bupropion-Containing Products: ®#®#
Laboratory Tests
Drug Interactions
max
Drugs Metabolized By Cytochrome P450IID6 (CYP2D6): max1/2
MAO Inhibitors: CONTRAINDICATIONS
Levodopa and Amantadine:
Drugs That Lower Seizure Threshold: WARNINGS
Nicotine Transdermal System: PRECAUTIONS: Cardiovascular Effects
Alcohol: CONTRAINDICATIONS
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Teratogenic Effects: 22
2
Labor and Delivery
Nursing Mothers
Pediatric Use
BOX WARNINGWARNINGS: Clinical Worsening and Suicide Risk in Treating Psychiatric Disorders
Geriatric Use
CLINICAL PHARMACOLOGY
PRECAUTIONS: Renal ImpairmentDOSAGE AND ADMINISTRATION
BUPROPION HYDROCHLORIDE ADVERSE REACTIONS
WARNINGSPRECAUTIONS
Incidence in Controlled Trials With Bupropion: Adverse Events Associated
With Discontinuation of Treatment Among Patients Treated With Bupropion Hydrochloride Extended-Release Tablets (SR):
| Adverse Event Term | Bupropion 300 mg/day (n = 376) |
Bupropion 400 mg/day (n = 114) |
Placebo (n = 385) |
|---|---|---|---|
| Rash Nausea Agitation Migraine |
2.4% 0.8% 0.3% 0.0% |
0.9% 1.8% 1.8% 1.8% |
0.0% 0.3% 0.3% 0.3% |
WARNINGSPRECAUTIONS
| Body System/Adverse Event | Bupropion 300 mg/day (n = 376) |
Bupropion 400 mg/day (n = 114) |
Placebo (n = 385) |
|---|---|---|---|
| Body (General) Headache Infection Abdominal pain Asthenia Chest pain Pain Fever |
26% 8% 3% 2% 3% 2% 1% |
25% 9% 9% 4% 4% 3% 2% |
23% 6% 2% 2% 1% 2%  |
| Cardiovascular Palpitation Flushing Migraine Hot flashes |
2% 1% 1% 1% |
6% 4% 4% 3% |
2%  1% 1% |
| Digestive Dry mouth Nausea Constipation Diarrhea Anorexia Vomiting Dysphagia |
17% 13% 10% 5% 5% 4% 0% |
24% 18% 5% 7% 3% 2% 2% |
7% 8% 7% 6% 2% 2% 0% |
| Musculoskeletal Myalgia Arthralgia Arthritis Twitch |
2% 1% 0% 1% |
6% 4% 2% 2% |
3% 1% 0%  |
| Nervous system Insomnia Dizziness Agitation Anxiety Tremor Nervousness Somnolence Irritability Memory decreased Paresthesia Central nervous system stimulation |
11% 7% 3% 5% 6% 5% 2% 3%  1% 2% |
16% 11% 9% 6% 3% 3% 3% 2% 3% 2% 1% |
6% 5% 2% 3% 1% 3% 2% 2% 1% 1% 1% |
| Respiratory Pharyngitis Sinusitis Increased cough |
3% 3% 1% |
11% 1% 2% |
2% 2% 1% |
| Skin Sweating Rash Pruritus Urticaria |
6% 5% 2% 2% |
5% 4% 4% 1% |
2% 1% 2% 0% |
| Special senses Tinnitus Taste perversion Blurred vision or diplopia |
6% 2% 3% |
6% 4% 2% |
2%  2% |
| Urogenital Urinary frequency Urinary urgency Vaginal hemorrhage  Urinary tract infection |
2%  0% 1% |
5% 2% 2% 0% |
2% 0%   |
Bupropion 300 mg/day:
Bupropion 400 mg/day:
Other Events Observed During the Clinical Development and Postmarketing Experience of Bupropion:
WARNINGSPRECAUTIONS
Body (General): PRECAUTIONS
Cardiovascular:
PRECAUTIONS
Digestive:
Endocrine:
Hemic and Lymphatic:
Metabolic and Nutritional:
Musculoskeletal:
Nervous System:
Respiratory:
Skin:
Special Senses:
Urogenital:
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class:
Humans:
Animals:
OVERDOSAGE
Human Overdose Experience:
Overdosage Management:
Physicians’ Desk Reference
BUPROPION HYDROCHLORIDE DOSAGE AND ADMINISTRATION
General Dosing Considerations: WARNINGS
Initial Treatment:
Increasing the Dosage Above 300 mg/day:
Maintenance Treatment: CLINICAL TRIALSCLINICAL PHARMACOLOGY
Dosage Adjustment for Patients with Impaired Hepatic Function: CLINICAL PHARMACOLOGYWARNINGSPRECAUTIONS
Dosage Adjustment for Patients with Impaired Renal Function: CLINICAL PHARMACOLOGYPRECAUTIONS
HOW SUPPLIED
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP.
#®
MEDICATION GUIDE
Bupropion Hydrochloride Extended-Release Tablets, USP (SR)
IMPORTANT: Be sure to read the three sections of this Medication Guide. The first section is about the risk of suicidal thoughts and actions with antidepressant medicines; the second section is about the risk of changes in thinking and behavior, depression and suicidal thoughts or actions with medicines used to quit smoking; and the third section is entitled “What Other Important Information Should I Know About Bupropion Hydrochloride Extended-Release Tablets (SR)?”
Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Talk to your, or your family member’s, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
- How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child’s healthcare provider for more information.
Quitting Smoking, Quit-Smoking Medications, Changes in Thinking and Behavior, Depression, and Suicidal Thoughts or Actions
This section of the Medication Guide is only about the risk of changes in thinking and behavior, depression and suicidal thoughts or actions with drugs used to quit smoking.
Although bupropion hydrochloride extended-release tablet (SR) is not a treatment for quitting smoking, it contains the same active ingredient (bupropion hydrochloride) as ZYBAN®* which is used to help patients quit smoking.
Some people have had changes in behavior, hostility, agitation, depression, suicidal thoughts or actions while taking bupropion to help them quit smoking. These symptoms can develop during treatment with bupropion or after stopping treatment with bupropion.
What Other Important Information Should I Know About Bupropion Hydrochloride Extended-Release Tablets (SR)?
Seizures: There is a chance of having a seizure (convulsion, fit) with bupropion hydrochloride extended-release tablets (SR), especially in people:
- with certain medical problems.
- who take certain medicines.
If you have a seizure while taking bupropion hydrochloride extended-release tablets (SR), stop taking the tablets and call your doctor right away.
- High blood pressure (hypertension). Some people get high blood pressure, that can be severe, while taking bupropion hydrochloride extended-release tablets (SR). The chance of high blood pressure may be higher if you also use nicotine replacement therapy (such as a nicotine patch) to help you stop smoking.
- Severe allergic reactions. Some people have severe allergic reaction to bupropion hydrochloride extended-release tablets (SR). Stop taking bupropion hydrochloride extended-release tablets (SR) and call your doctor right away if you get a rash, itching, hives, fever, swollen lymph glands, painful sores in the mouth or around the eyes, swelling of the lips or tongue, chest pain, or have trouble breathing. These could be signs of a serious allergic reaction.
- Unusual thoughts or behaviors. Some patients have unusual thoughts or behaviors while taking bupropion hydrochloride extended-release tablets (SR), including delusions (believe you are someone else), hallucinations (seeing or hearing things that are not there), paranoia (feeling that people are against you), or feeling confused. If this happens to you, call your doctor.
Who should not take bupropion hydrochloride extended-release tablets (SR)?
Do not take bupropion hydrochloride extended-release tablets (SR) if you
- have or had a seizure disorder or epilepsy.
- are taking ZYBAN®* (used to help people stop smoking) or any other medicines that contain bupropion hydrochloride, such as WELLBUTRIN®* Tablets or WELLBUTRIN XL®* Extended-Release Tablets. Bupropion is the same active ingredient that is in bupropion hydrochloride extended-release tablets (SR).
- drink a lot of alcohol and abruptly stop drinking, or use medicines called sedatives (these make you sleepy) or benzodiazepines and you stop using them all of a sudden.
- have taken within the last 14 days medicine for depression called a monoamine oxidase inhibitor (MAOI), such as NARDIL®* (phenelzine sulfate), PARNATE®* (tranylcypromine sulfate), or MARPLAN®* (isocarboxazid).
- have or had an eating disorder such as anorexia nervosa or bulimia.
- are allergic to the active ingredient in bupropion hydrochloride extended-release tablets (SR), bupropion, or to any of the inactive ingredients. See the end of this leaflet for a complete list of ingredients in bupropion hydrochloride extended-release tablets (SR).
a. Tell your doctor about your other medical conditions including if you:
- are pregnant or plan to become pregnant. It is not known if bupropion hydrochloride extended-release tablets (SR) can harm your unborn baby.
- are breastfeeding. Bupropion passes through your milk. It is not known if bupropion hydrochloride extended-release tablets (SR) can harm your baby.
- have liver problems, especially cirrhosis of the liver.
- have kidney problems.
- have an eating disorder such as anorexia nervosa or bulimia.
- have had a head injury.
- have had a seizure (convulsion, fit).
- have a tumor in your nervous system (brain or spine).
- have had a heart attack, heart problems, or high blood pressure.
- are a diabetic taking insulin or other medicines to control your blood sugar.
- drink a lot of alcohol.
- abuse prescription medicines or street drugs.
How should I take bupropion hydrochloride extended-release tablets (SR)?
- Take bupropion hydrochloride extended-release tablets (SR) exactly as prescribed by your doctor.
- Do not chew, cut, or crush bupropion hydrochloride extended-release tablets (SR). You must swallow the tablets whole. Tell your doctor if you cannot swallow medicine tablets.
- Take bupropion hydrochloride extended-release tablets (SR) at the same time each day.
- Take your doses of bupropion hydrochloride extended-release tablets (SR) at least 8 hours apart.
- You may take bupropion hydrochloride extended-release tablets (SR) with or without food.
- If you miss a dose, do not take an extra tablet to make up for the dose you forgot. Wait and take your next tablet at the regular time. This is very important. Too much bupropion hydrochloride extended-release tablets (SR) can increase your chance of having a seizure.
- If you take too much bupropion hydrochloride extended-release tablets (SR), or overdose, call your local emergency room or poison control center right away.
- Do not take any other medicines while using bupropion hydrochloride extended-release tablets (SR) unless your doctor has told you it is okay.
- It may take several weeks for you to feel that bupropion hydrochloride extended-release tablet (SR) is working. Once you feel better, it is important to keep taking bupropion hydrochloride extended-release tablets (SR) exactly as directed by your doctor. Call your doctor if you do not feel bupropion hydrochloride extended-release tablet (SR) is working for you.
- Do not change your dose or stop taking bupropion hydrochloride extended-release tablets (SR) without talking with your doctor first.
What should I avoid while taking bupropion hydrochloride extended-release tablets (SR)?
- Do not drink a lot of alcohol while taking bupropion hydrochloride extended-release tablets (SR). If you usually drink a lot of alcohol, talk with your doctor before suddenly stopping. If you suddenly stop drinking alcohol, you may increase your chance of having seizures.
- Do not drive a car or use heavy machinery until you know how bupropion hydrochloride extended-release tablet (SR) affects you. Bupropion hydrochloride extended-release tablets (SR) can impair your ability to perform these tasks.
What are possible side effects of bupropion hydrochloride extended-release tablets (SR)?
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store bupropion hydrochloride extended-release tablets (SR)?
- Store bupropion hydrochloride extended-release tablets (SR) at room temperature. Store out of direct sunlight. Keep bupropion hydrochloride extended-release tablets (SR) in its tightly closed bottle.
- Bupropion hydrochloride extended-release tablets (SR) may have an odor.
What are the ingredients in bupropion hydrochloride extended-release tablets (SR)?
®®®®®®
Rx only
Distributed by:
Caraco Pharmaceutical Laboratories, Ltd.
1150 Elijah McCoy Drive, Detroit, MI 48202
Manufactured at:
Sun Pharmaceutical Ind. Ltd.
Halol-Baroda Highway,
Halol-389 350, Gujarat, India.
ISS. 04/2010
PJPI0292
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 100MG
NDC 47335--736-86
buPROPion Hydrochloride Extended-Release Tablets, USP (SR)
100 mg
Twice-A-Day
Warning: Do not use in combination with ZYBAN®, or any other medicines that contain bupropion hydrochloride.
Rx only
60 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.
PHARMACIST: PLEASE DISPENSE WITH MEDICATION GUIDE

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 150MG
NDC 47335--737-86
buPROPion Hydrochloride Extended-Release Tablets, USP (SR)
150 mg
Twice-A-Day
Warning: Do not use in combination with ZYBAN®, or any other medicines that contain bupropion hydrochloride.
Rx only
60 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.
PHARMACIST: PLEASE DISPENSE WITH MEDICATION GUIDE

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 200MG
NDC 47335--738-86
buPROPion Hydrochloride Extended-Release Tablets, USP (SR)
200 mg
Twice-A-Day
Warning: Do not use in combination with ZYBAN®, or any other medicines that contain bupropion hydrochloride.
Rx only
60 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.
PHARMACIST: PLEASE DISPENSE WITH MEDICATION GUIDE

Bupropion hydrochlorideBupropion hydrochloride TABLET, FILM COATED, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Bupropion hydrochlorideBupropion hydrochloride TABLET, FILM COATED, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Bupropion hydrochlorideBupropion hydrochloride TABLET, FILM COATED, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||