Buminate
BUMINATE 25%, Albumin (Human), USP, 25% Solution
FULL PRESCRIBING INFORMATION: CONTENTS*
- BUMINATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- BUMINATE INDICATIONS AND USAGE
- BUMINATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- BUMINATE ADVERSE REACTIONS
- OVERDOSE
- BUMINATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
- REFERENCES
- Principal Display Panel
FULL PRESCRIBING INFORMATION
BUMINATE DESCRIPTION
BUMINATE 25%, in 20, 50 and 100 mL glass bottles is a sterile, nonpyrogenic preparation of albumin in a single dosage form for intravenous administration. Each 100 mL contains 25 g of albumin and was prepared from human venous plasma using the Cohn cold ethanol fractionation process. Source material for fractionation may be obtained from another U.S. licensed manufacturer. It has been adjusted to physiological pH with sodium bicarbonate and/or sodium hydroxide and stabilized with N-acetyltryptophan (0.02M) and sodium caprylate (0.02M). The sodium content is 145 ± 15 mEq/L. This solution contains no preservative and none of the coagulation factors found in fresh whole blood or plasma. BUMINATE 25%, is a transparent or slightly opalescent solution which may have a greenish tint or may vary from a pale straw to an amber color.
The likelihood of the presence of viable hepatitis viruses has been minimized by testing the plasma at three stages for the presence of hepatitis viruses, by fractionation steps with demonstrated virus removal capacity and by heating the product for 10 hours at 60°C. This procedure has been shown to be an effective method of inactivating hepatitis virus in albumin solutions even when those solutions were prepared from plasma known to be infective.1-3
CLINICAL PHARMACOLOGY
Albumin is responsible for 70-80% of the colloid osmotic pressure of normal plasma, thus making it useful in regulating the volume of circulating blood.4-6 Albumin is also a transport protein and binds naturally occurring, therapeutic and toxic materials in the circulation.5,6
BUMINATE 25% is osmotically equivalent to approximately five times its volume of human plasma. When injected intravenously, 25% albumin will draw about 3.5 times its volume of additional fluid into the circulation within 15 minutes, except when the patient is markedly dehydrated. This extra fluid reduces hemoconcentration and blood viscosity. The degree and duration of volume expansion depends upon the initial blood volume. With patients treated for diminished blood volume, the effect of infused albumin may persist for many hours; however, in patients with normal volume, the duration will be shorter.7-9
Total body albumin is estimated to be 350 g for a 70 kg man and is distributed throughout the extracellular compartments; more than 60% is located in the extravascular fluid compartment. The half-life of albumin is 15 to 20 days with a turnover of approximately 15 g per day.5
The minimum plasma albumin level necessary to prevent or reverse peripheral edema is unknown. Some investigators recommend that plasma albumin levels be maintained at approximately 2.5 g/dL. This concentration provides a plasma oncotic value of 20 mm Hg.4
BUMINATE 25% is manufactured from human plasma by the modified Cohn-Oncley cold ethanol fractionation process, which includes a series of cold-ethanol precipitation, centrifugation and/or filtration steps followed by pasteurization of the final product at 60 ± 0.5°C for 10 - 11 hours. This process accomplishes both purification of albumin and reduction of viruses.
In vitro studies demonstrate that the manufacturing process for BUMINATE 25% provides for significant viral reduction. These viral reduction studies, summarized in Table 1, demonstrate viral clearance during the manufacturing process for BUMINATE 25% using human immunodeficiency virus, type 1 (HIV-1) both as a target virus and as model virus for HIV-2 and other lipid-enveloped RNA viruses; bovine viral diarrhea virus (BVDV), a model for lipid-enveloped RNA viruses, such as hepatitis C virus (HCV); West Nile Virus (WNV), a target virus and model for other similar lipid-enveloped RNA viruses; pseudorabies virus (PRV), a model for other lipid-enveloped DNA viruses such as hepatitis B virus (HBV); mice minute virus (MMV), models for non-enveloped DNA viruses such as human parvovirus B1910 ; hepatitis A virus (HAV), a target virus and a model for other non-enveloped RNA viruses.
These studies indicate that specific steps in the manufacture of BUMINATE 25% are capable of eliminating/inactivating a wide range of relevant and model viruses. Since the mechanism of virus elimination/inactivation by fractionation and by heating steps is different, the overall manufacturing process of BUMINATE 25% is robust in reducing viral load.
|
Table 1 Summary of Viral Reduction Factor for Each Virus and Processing Step |
||||||
| Process Step | Viral Reduction Factor (log10) | |||||
| Lipid Enveloped | Non Enveloped | |||||
| HIV-1 | Flaviviridae | PRV | HAV | Parvoviridae | ||
| BVDV | WNV | MMV | ||||
| Processing of
Fraction I+II+III/II+III supernatant to Fraction
IV4 Cuno 70C filtrate |
>4.9 | >4.8 | >5.7 | >5.5 | >4.5 | 3.0 |
| Pasteurization | >7.8 | >6.5 |
n.d. |
>7.4 | 3.2 |
1.6 |
| Mean Cumulative Reduction Factor, log10 | >12.7 | >11.3 | >5.7 | >12.9 | >7.7 | 4.6 |
BUMINATE INDICATIONS AND USAGE
1. Hypovolemia
Hypovolemia is a possible indication for BUMINATE 25%. Its effectiveness in reversing hypovolemia depends largely upon its ability to draw interstitial fluid into the circulation. It is most effective with patients who are well hydrated. When hypovolemia is long standing and hypoalbuminemia exists accompanied by adequate hydration or edema, 25% albumin is preferable to 5% protein solutions.4,6 However, in the absence of adequate or excessive hydration, 5% protein solutions should be used or 25% albumin should be diluted with crystalloid solutions.
Although crystalloid solutions and colloid-containing plasma substitutes can be used in emergency treatment of shock, Albumin (Human) has a prolonged intravascular half-life.11 When blood volume deficit is the result of hemorrhage, compatible red blood cells or whole blood should be administered as quickly as possible.
2. Hypoalbuminemia
Hypoalbuminemia is another possible indication for use of BUMINATE 25%. Hypoalbuminemia can result from one or more of the following:5
- Inadequate production (malnutrition, burns, major injury, infections, etc.)
- Excessive catabolism (burns, major injury, pancreatitis, etc.)
- Loss from the body (hemorrhage, excessive renal excretion, burn exudates, etc.)
- Redistribution within the body (major surgery, various inflammatory conditions, etc.)
When albumin deficit is the result of excessive protein loss, the effect of administration of albumin will be temporary unless the underlying disorder is reversed. In most cases, increased nutritional replacement of amino acids and/or protein with concurrent treatment of the underlying disorder will restore normal plasma albumin levels more effectively than albumin solutions. Occasionally hypoalbuminemia accompanying severe injuries, infections or pancreatitis cannot be quickly reversed and nutritional supplements may fail to restore serum albumin levels. In these cases, BUMINATE 25% might be a useful therapeutic adjunct.
An optimum regimen for the use of albumin, electrolytes and fluid in the early treatment of burns has not been established, however, in conjunction with appropriate crystalloid therapy, BUMINATE 25% may be indicated for treatment of oncotic deficits after the initial 24 hour period following extensive burns and to replace the protein loss which accompanies any severe burn.4,6
A characteristic of ARDS is a hypoproteinemic state, which may be causally related to the interstitial pulmonary edema. Although uncertainty exists concerning the precise indication of albumin infusion in these patients, if there is a pulmonary overload accompanied by hypoalbuminemia, 25% albumin solution may have a therapeutic effect when used with a diuretic.4
BUMINATE 25% may be a useful aid in treating edema in patients with severe nephrosis who are receiving steroids and/or diuretics.
3. Cardiopulmonary Bypass Surgery
BUMINATE 25% has been recommended prior to or during cardiopulmonary bypass surgery, although no clear data exist indicating its advantage over crystalloid solutions.4,6,12
4. Hemolytic Disease of the Newborn (HDN)
BUMINATE 25% may be administered in an attempt to bind and detoxify unconjugated bilirubin in infants with severe HDN.
There is no valid reason for use of albumin as an intravenous nutrient.
BUMINATE CONTRAINDICATIONS
A history of allergic reactions to albumin and any of the excipients is a specific contraindication to the use of this product.
BUMINATE 25% is also contraindicated in severely anemic patients and in patients with cardiac failure.
BUMINATE 25% must not be diluted with Sterile Water for Injection as this may cause hemolysis in recipients. There exists a risk of potentially fatal hemolysis and acute renal failure from the use of Sterile Water for Injection as a diluent for Albumin (Human). Acceptable diluents include 0.9% Sodium Chloride or 5% Dextrose in Water.
BUMINATE 25% must not be administered to patients with chronic renal insufficiencies due to the potential for accumulations of aluminum. Accumulations of aluminum in patients with chronic renal insufficiencies have led to toxic manifestations such as hypercalcemia, vitamin D-refractory osteodystrophy, anemia, and severe progressive encephalopathy.13-15, 19
WARNINGS
BUMINATE 25% is made from human plasma. Products made from human plasma may contain infectious agents, such as viruses, that can cause disease. This also applies to unknown or emerging viruses and pathogens. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and/or removing certain viruses (See Description ). The measures taken are considered effective for enveloped viruses such as HIV, HBV, and HCV, and for the non-enveloped viruses HAV and Parvovirus B19. Despite these measures, such products can still potentially transmit disease. Based on effective donor screening and product manufacturing processes, albumin carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin. ALL infections thought by a physician possibly to have been transmitted by this product, should be reported by the physician, or other healthcare provider to Baxter Healthcare Corporation at 1-800-423-2862. The physician should discuss the risks and benefits of this product with the patient.
Suspicion of allergic or anaphylactic type reactions requires immediate discontinuation of the injection. In case of shock, standard medical treatment for shock should be implemented.
PRECAUTIONS
Certain components used in the packaging of this product contain natural rubber latex.
Hemodynamics
Do not administer BUMINATE 25% without very close monitoring of hemodynamics; look for evidence of cardiac or respiratory failure, renal failure, or increasing intra-cranial pressure.
Hypervolemia/Hemodilution
BUMINATE 25% should be used with caution in conditions where hypervolemia and its consequences or hemodilution could represent a special risk for the patient. Examples may include but are not limited to: decompensated cardiac insufficiency, hypertension, esophageal varices, pulmonary edema, hemorrhagic diathesis, severe anemia, renal and post-renal failure.
BUMINATE 25% must be administered intravenously. The rate of administration should be adjusted according to the solution concentration and the patient’s hemodynamic measurements and should not exceed 1mL/min to patients with normal blood volume. More rapid administration might cause circulatory overload and pulmonary edema.16 At the first clinical signs of cardiovascular overload (headache, dyspnea, jugular vein congestion), or increased blood pressure, raised central venous pressure and pulmonary edema, the infusion is to be stopped immediately.
Blood Pressure
A rise in blood pressure after 25% albumin infusion necessitates careful observation of the injured or post-operative patient in order to detect and treat severed blood vessels that may not have bled at a lower blood pressure.
Pregnancy–Category C, and Lactation
There are no adequate data from the use of BUMNIATE 25% in pregnant or lactating women. Animal reproduction studies have not been conducted with BUMINATE 25% . It is not known whether BUMINATE 25% can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Physicians should carefully consider the potential risks and benefits for each specific patient before prescribing BUMINATE 25%. BUMINATE 25% should be given to a pregnant woman only if clearly needed.
Pediatric Use
The safety of albumin solutions has been demonstrated in children provided the dose is appropriate for body weight, however, the safety of BUMINATE 25% has not been evaluated in pediatric patients.
Large Volumes
If comparatively large volumes are to be replaced, controls of coagulation and hematocrit are necessary. Care must be taken to ensure adequate substitution of other blood constituents (coagulation factors, electrolytes, platelets, and erythrocytes). Appropriate hemodynamic monitoring should be undertaken.
Electrolyte Status
When BUMINATE 25% is given, the electrolyte status of the patient should be monitored and appropriate steps taken to restore or maintain the electrolyte balance.
DRUG INTERACTIONS
No interaction studies have been performed with BUMINATE 25%.
BUMINATE ADVERSE REACTIONS
Side Effects from Clinical Trials
There are no data available on adverse reactions from clinical trials conducted with BUMINATE 25%.
Post-Marketing Side Effects
The following adverse reactions have been reported in the post-marketing experience. These reactions are listed by MedDRA System Organ Class (SOC), then by Preferred Term in order of severity.
IMMUNE SYSTEM DISORDERS: Anaphylactic shock, Anaphylactic reactions, Hypersensitivity/Allergic reactions
NERVOUS SYSTEM DISORDERS: Headache
CARDIAC DISORDERS: Tachycardia
VASCULAR DISORDERS: Hypotension, Flushing
RESPIRATORY, THORACIC, AND MEDIASTINAL DISORDERS: Dyspnea
GASTROINTESTINAL DISORDERS: Vomiting, Nausea, Dysguesia
SKIN AND SUBCUTANEOUS TISSUE DISORDERS: Urticaria, Rash, Pruritis
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS: Pyrexia, Chills
OVERDOSE
Hypervolemia may occur if the dosage and rate of infusion are too high. (See Precautions: Hypervolemia/Hemodilution)
BUMINATE DOSAGE AND ADMINISTRATION
BUMINATE 25% must be administered intravenously. Do not use if turbid. Do not begin administration more than 4 hours after the container has been entered. Discard unused portion.
BUMINATE 25% solutions must not be diluted with Sterile Water for Injection as this may cause hemolysis in recipients (see CONTRAINDICATIONS).
Albumin solutions should not be mixed with other medicinal products including blood and blood components, but can be used concomitantly with other parenterals such as whole blood, plasma, saline, glucose or sodium lactate when deemed medically necessary. The addition of four volumes of normal saline or 5% glucose to 1 volume of BUMINATE 25% gives a solution, which is approximately isotonic and isosmotic with citrated plasma.
Albumin solutions should not be mixed with protein hydrolysates or solutions containing alcohol since these combinations may cause the proteins to precipitate.
Do not add supplementary medication.
Hypervolemia may occur if the dosage and rate of infusion are not adjusted, giving consideration to the solution concentration and the patient’s clinical status. Hemodynamic parameters should be monitored in patients receiving BUMINATE 25% and should be used to check for the risk of hypervolemia and cardiovascular overload. (See PRECAUTIONS: Hypervolemia/Hemodilution).
It is strongly recommended that every time that BUMINATE 25% is administered to a patient, the name and batch number of the product be recorded in order to maintain a link between the patient and the batch of the product.
Recommended Dosages
1. Hypovolemic Shock
The dosage of BUMINATE 25% must be individualized. As a guideline, the initial treatment should be in the range of 100 to 200 mL for adults and 2.5 to 5 mL per kilogram body weight for children. This may be repeated after 15 to 30 minutes, if the response is not adequate. For patients with significant plasma volume deficits, albumin replacement is best administered in the form of 5% Albumin (Human).
Upon administration of additional albumin or if hemorrhage has occurred, hemodilution and a relative anemia will follow. This condition should be controlled by the supplemental administration of compatible red blood cells or compatible whole blood.
2. Burns
The optimal therapeutic regimen for administration of crystalloid and colloid solutions after extensive burns has not been established. When BUMINATE 25% is administered after the first 24 hours following burns, the dose should be determined according to the patient’s condition and response to treatment.
3. Hypoalbuminemia
Hypoalbuminemia is usually accompanied by a hidden extravascular albumin deficiency of equal magnitude. This total body albumin deficit must be considered when determining the amount of albumin necessary to reverse the hypoalbuminemia. When using patient’s serum albumin concentration to estimate the deficit, the body albumin compartment should be calculated to be 80 to100 mL per kg of body weight.5,6 Daily dose should not exceed 2 g of albumin per kilogram of body weight.
4. Hemolytic Disease of the Newborn
BUMINATE 25% may be administered prior to or during exchange transfusion in a dose of 1 g per kilogram body weight.17
Preparation for Administration
Do not use unless solution is clear of particulate matter and seal is intact. BUMNATE 25% is a transparent or slightly opalescent solution, which may have a greenish tint or may vary from a pale straw to an amber color. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Remove cap from bottle to expose center portion of rubber stopper
- Clean stopper with germicidal solution.
Administration
Follow directions for use printed on the administration set container. Make certain that the administration set contains an adequate filter (15-micron or smaller).
HOW SUPPLIED
BUMINATE 25% is supplied in 20 mL (NDC 0944-0490-01), 50 mL (NDC 0944-0490-02) and 100 mL (NDC 0944-0490-03) bottles.
STORAGE
Store BUMINATE 25% at room temperature, not to exceed 30°C (86°F). Avoid freezing to prevent damage to the bottle.
Stability testing for BUMINATE 25% showed that aluminum concentration increased over time reaching levels that could exceed 1000 ppb over the shelf life of the product. (See CONTRAINDICATIONS).18, 19
REFERENCES
- Cai K, Gierman T, et al: Ensuring the Biologic Safety of Plasma-Derived Therapeutic Proteins. Department of Preclinical Research and Pathogen Safety, Bayer HealthCare LLC, North Carolina, USA. Biodrugs 19(2): 79-96 2005.
- Gerety RJ, Aronson DL: Plasma derivatives and viral hepatitis. Transfusion 22:347 351, 1982
- Burnouf T, Padilla A, Current strategies to prevent transmission of prions by human plasma derivatives. Transfusion Clinique et Biologique 13: 320-328, 2006
- Tullis JL: Albumin, 1. Background and use, and 2. Guidelines for clinical use. JAMA 237:355-360, 460-463, 1977
- Peters T Jr: Serum albumin, in The Plasma Proteins, 2nd ed, Vol 1. Putnam FW (ed). New York, Academic Press, 1975, pp 133-181
- Finlayson JS: Albumin products. Semin Thromb Hemostas. 6:85-120, 1980
- Haynes G, Navickis R, Wilkes M, Albumin administration – what is the evidence of clinical benefit? Asystematic review of randomized controlled trials. European Journey of Anesthesiology 20:771-793 2003
- Mendez, C, McClain C, Marsano L et al, Albumin Therapy in Clinical Practice. Nutrition in Clinical Practice 20 No. 3:314-320, June 2005
- Quinlan G, Martin G, Evans T, Albumin: Biochemical Properties and Therapeutic Potential. HEMATOLOGY Vol 14, No. 6 1211-1219 2005
- J. Blümel et al., Inactivation of Parvovirus B19 During Pasteurization of Human Serum Albumin. Transfusion 42:1011-1018 , 2002
- Shoemaker WC, Schluchter M, Hopkins JA, et al: Comparison of the relative effectiveness of colloids and crystalloids in emergency resuscitation. Am J Surg 142:73 83, 1981
- Lowenstein E, Hallowell P, Bland JHL: Use of colloid and crystalloid solutions in open heart surgery: Physiological basis and clinical results in, Proceedings of the Workshop on Albumin. Sgouris JT, Rene A (eds). DHEW Publication No. (NIH) 76-925, Washington, DC, US Government Printing Office, 1976, pp 195-210.
- Milliner DS, Shenaberger JH, Shuman P, et al: Inadvertent aluminum administration during plasma exchange due to aluminum contamination of albumin-replacement solutions. N Engl J Med 312:165-7,1985
- Ott SM, Maloney NA, Klein GL, et al: Aluminum is associated with low bone formation in patients receiving chronic parenteral nutrition. Ann Intern Med 98:910-4,1983
- Wills MR, Savory J: Aluminum poisoning: dialysis encephalopathy, osteomalacia, and anaemia. Lancet 2:29-34,1983
- Grocott, Michael P.W., Mythen, Michael G., and Gan, Tong J. Perioperative Fluid Management and Clinical Outcomes in Adults. Anesth Analg. 2005;100:1100
- Tsao YC, Yu VYH: Albumin in management of neonatal hyperbilirubinaemia. Arch Dis Childhood 47:250-256, 1972
- Data on file; Baxter Healthcare Corporation
- Data on file; Baxter Healthcare Corporation
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
To enroll in the confidential industry-wide Patient Notification System, call 1-888-UPDATE U (1-888-873-2838).
Baxter and Buminate are trademarks of Baxter International Inc.
Revised Sept 2009
Principal Display Panel
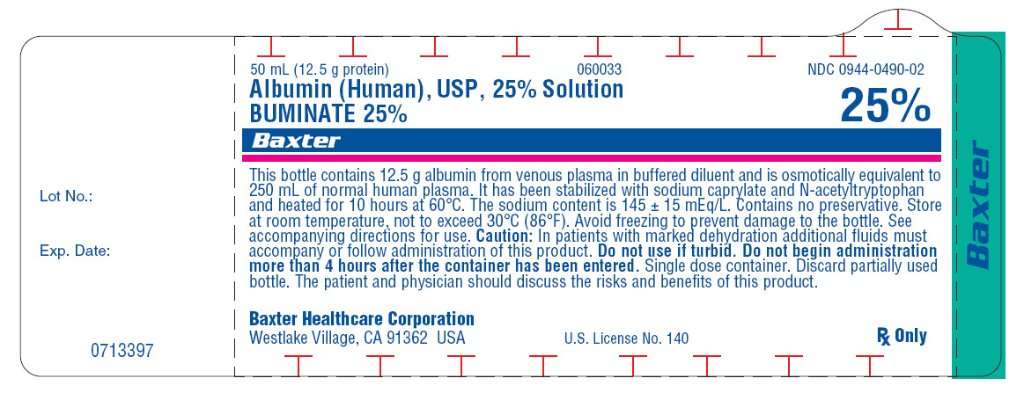
50 mL (12.5 g protein)
NDC 0944-0490-02
Albumin (Human), USP, 25% Solution
BUMINATE 25%
This bottle contains 12.5 g albumin from venous plasma in buffered diluent and is osmotically equivalent to 250 mL of normal human plasma. It has been stabilized with sodium caprylate and sodium acetyltryptophanate and heated for 10 hours at 60ºC. The sodium content is 145 ± 15 mEq/L. Contains no preservative. Store at room temperature, not to exceed 30ºC (86ºF). Avoid freezing to prevent damage to the bottle. See accompanying directions for use.
Caution: In patients with marked dehydration additional fluids must accompany or follow administration of this product. Do not use if turbid. Do not begin administration more than 4 hours after the container has been entered. Single dose container. Discard partially used bottle.
The patient and physician should discuss the risks and benefits of this product.
Rx Only
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
US. License No. 140
BuminateAlbumin Human INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||