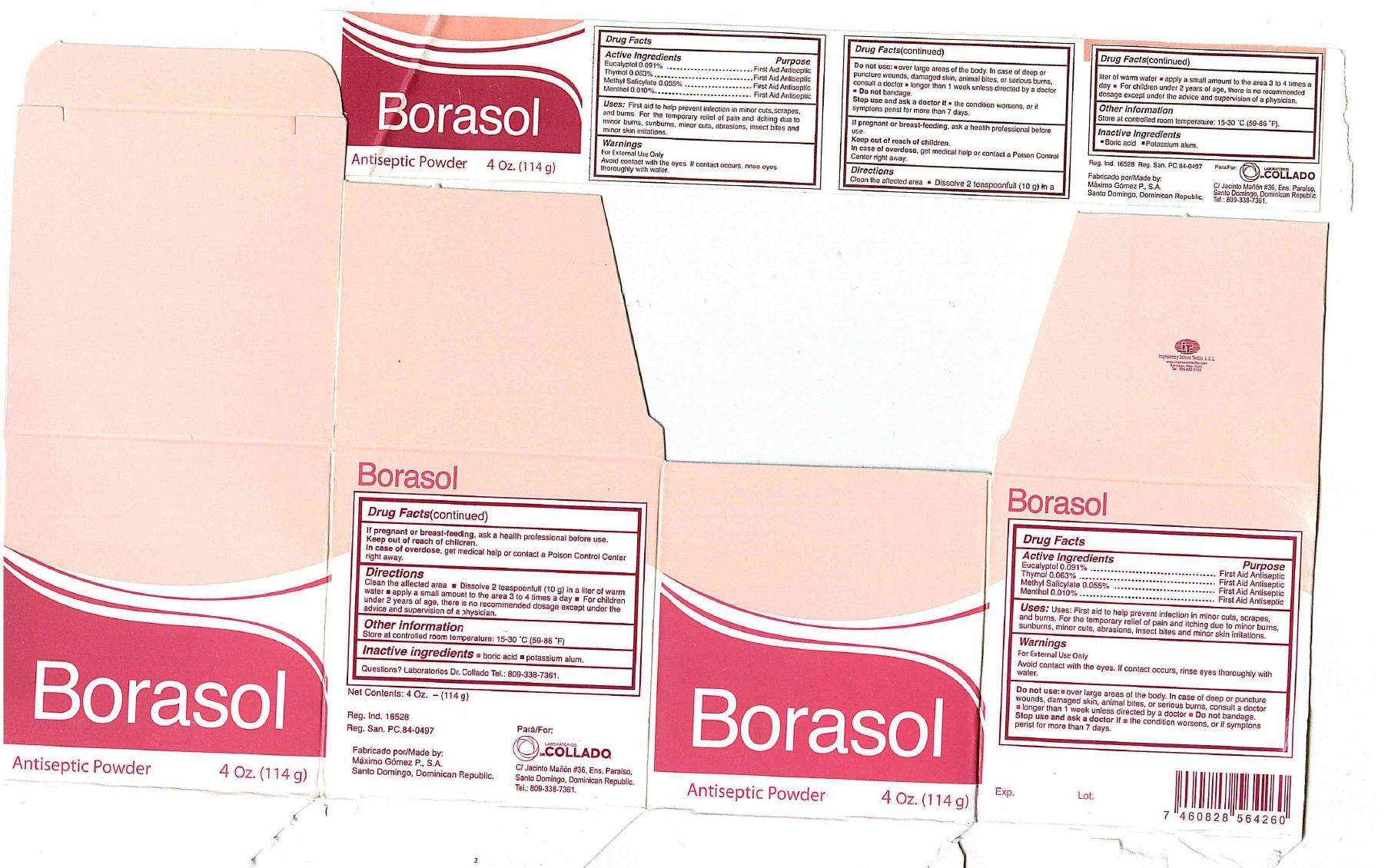
Borasol
Laboratorios Dr. Collado, C x A.
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients Purpose
Eucalyptol 0.091% First Aid Antiseptic
Thymol 0.063% First Aid Antiseptic
Methyl Salicylate 0.055% First Aid Antiseptic
Menthol 0.010% First Aid Antiseptic
Purpose
Purpose
First Aid Antiseptic
Warnings
For external use only
Avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water.
Do not use:
- over large areas of the body. In case of deep or puncture wounds, damaged skin, animal bites, or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
- Do not bandage.
Stop use and ask a doctor if
- the condition worsens, or if symptoms persist for more than 7 days
If pregnant or breast-feeding, ask a health professional before use
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- Clean the affected area
- Dissolve 2 teasponnfull (10g) in a liter of warm water
- apply a small amount to the area 3 to 4 times a day
- For children under 2 years of agea, there is no recommended dosage except under the advice and supervision of a physician
Uses
Uses:First aid to help prevent infection in minor cuts, scrapes, and burns. For the temporary relief of pain and itching due to minor burns, sunburns, minor cuts, abrasions, insect bites and minor skin irritations.
Other information
Store at controlled room temperature: 15-30 degrees C (59-86 degrees F)
Inactive ingredients
boric acid, potassium alum
Questions?Laboratorios Dr. Collado Tel: 809-338-7361
BorasolEucalyptol, Thymol, Methyl Salicylate, Menthol POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||