Body Balance
Energique, Inc.
Apotheca Company
DRUG FACTS:
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS:
- INDICATIONS:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE DISPLAY LABEL:
FULL PRESCRIBING INFORMATION
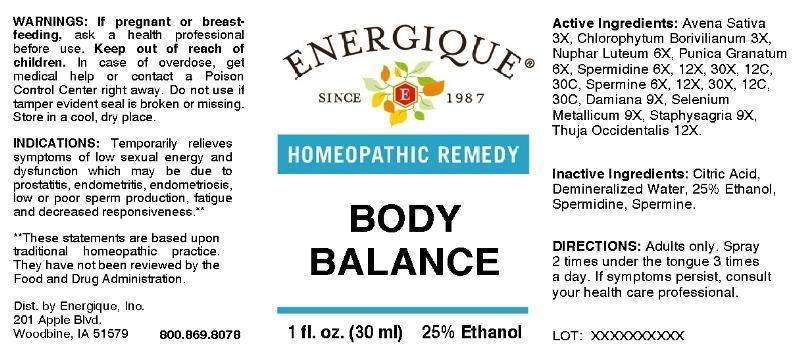
ACTIVE INGREDIENTS:
Avena Sativa 3X, Chlorophytum Borivilianum 3X, Nuphar Luteum 6X, Punica Granatum (Fruit) 6X, Spermidine 6X, 12X, 30X, 12C, 30C, Spermine 6X, 12X, 30X, 12C, 30C, Damiana 9X, Selenium Metallicum 9X, Staphysagria 9X, Thuja Occidentalis 12X.
INDICATIONS:
Temporarily relieves symptoms of low sexual energy and dysfunction which may be due to prostatitis, endometritis, endometriosis, low or poor sperm production, fatigue and decreased responsiveness.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
WARNINGS:
If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Do not use if tamper evident seal is broken or missing. Store in a cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Adults only. Spray 2 times under the tongue 3 times a day. If symptoms persist, consult your health care professional.
INDICATIONS:
Temporarily relieves symptoms of low sexual energy and dysfunction which may be due to prostatitis, endometritis, endometriosis, low or poor sperm production, fatigue and decreased responsiveness.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
INACTIVE INGREDIENTS:
Citric Acid, Demineralized Water, 25% Ethanol, Spermidine, Spermine.
QUESTIONS:
Dist. by Energique, Inc.
201 Apple Blvd.
Woodbine, IA 51579 800.869.8078
PACKAGE DISPLAY LABEL:
ENERGIQUE
HOMEOPATHIC REMEDY
BODY BALANCE
1 fl. oz. (30 ml) 25% Ethanol
Body BalanceAvena Sativa, Chlorophytum Borivilianum, Nuphar Luteum, Punica Granatum, Spermidine, Spermine, Damiana, Selenium Metallicum, Staphysagria, Thuja Occidentalis LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||