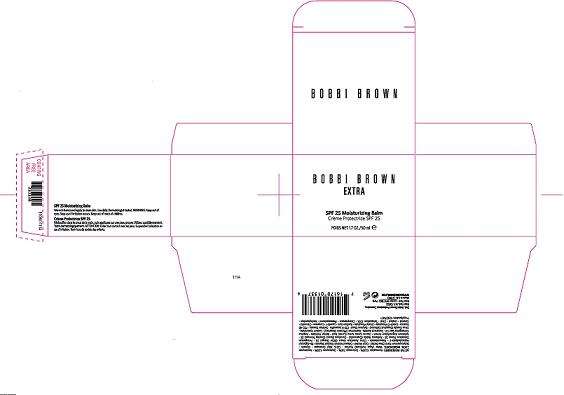
BOBBI BROWN EXTRA
BOBBI BROWN PROFESSIONAL COSMETICS INC
FULL PRESCRIBING INFORMATION
Active ingredient
ACTIVE INGREDIENTS: HOMOSALATE 10.00% [] OCTINOXATE 7.50% [] OXYBENZONE 5.00% [] AVOBENZONE 2.00%
WARNING: KEEP OUT OF EYES. STOP USE IF IRRITATION OCCURS.
DIRECTIONS: WARM IN HANDS AND APPLY TO CLEAN SKIN
PRINCIPAL DISPLAY PANEL:
BOBBI BROWN
EXTRA
SPF 25 MOISTURIZING BALM
BOBBI BROWN EXTRAHOMOSALATE, OCTINOXATE, OXYBENZONE, AVOBENZONE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!