Bloxiverz
HIGHLIGHTS OF PRESCRIBING INFORMATION INDICATIONS AND USAGEBLOXIVERZ, a cholinesterase inhibitor, is indicated for the reversal of the effects of non-depolarizing neuromuscular blocking agents (NMBAs) after surgery (1).DOSAGE AND ADMINISTRATION Should be administered by trained healthcare providers (2.1) Peripheral nerve stimulator and monitoring for twitch responses should be used to determine when BLOXIVERZ should be initiated and if additional doses are needed (2.2) -For reversal of NMBAs with shorter half-lives, when first twitch response is substantially greater than 10% of baseline, or when a second twitch is present: 0.03 mg/kg by intravenous route (2.2) -For reversal of NMBAs with longer half-lives or when first twitch response is close to 10% of baseline: 0.07 mg/kg by intravenous route (2.2) Maximum total dosage is 0.07 mg/kg or up to a total of 5 mg (whichever is less) (2.2) An anticholinergic agent, e.g., atropine sulfate or glycopyrrolate, should be administered prior to or concomitantly with BLOXIVERZ (2.4) DOSAGE FORMS AND STRENGTHSInjection: 0.5 mg/mL and 1 mg/mL in 10 mL multiple-dose vials (3)CONTRAINDICATIONS Hypersensitivity to neostigmine (4) Peritonitis or mechanical obstruction of the intestinal or urinary tract (4) WARNINGS AND PRECAUTIONS Bradycardia: Atropine or glycopyrrolate should be administered prior to BLOXIVERZ to lessen risk of bradycardia. (5.1) Serious Reactions with Coexisting Conditions: Use with caution in patients with, coronary artery disease, cardiac arrhythmias, recent acute coronary syndrome or myasthenia gravis. (5.2) Neuromuscular Dysfunction: Can occur if large doses of BLOXIVERZ are administered when neuromuscular blockade is minimal; reduce dose if recovery from neuromuscular blockade is nearly complete. (5.4) Side EffectsMost common adverse reactions during treatment: bradycardia, nausea and vomiting. (6) To report SUSPECTED ADVERSE REACTIONS, contact Éclat Pharmaceuticals at 1-877-622-2320 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. USE IN SPECIFIC POPULATIONS Pregnancy: No human or animal data. Use only if clearly needed.
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1. BLOXIVERZ INDICATIONS AND USAGE
- 2. BLOXIVERZ DOSAGE AND ADMINISTRATION
- 3. DOSAGE FORMS AND STRENGTHS
- 4. BLOXIVERZ CONTRAINDICATIONS
- 5. WARNINGS AND PRECAUTIONS
- 6. BLOXIVERZ ADVERSE REACTIONS
- 7. DRUG INTERACTIONS
- 8. USE IN SPECIFIC POPULATIONS
- 10. OVERDOSAGE
- 11. BLOXIVERZ DESCRIPTION
- 12. CLINICAL PHARMACOLOGY
- 13. NONCLINICAL TOXICOLOGY
- 14. CLINICAL STUDIES
- 16. HOW SUPPLIED/STORAGE AND HANDLING
FULL PRESCRIBING INFORMATION
1. INDICATIONS AND USAGE
BLOXIVERZ is a cholinesterase inhibitor indicated for the reversal of the effects of non-depolarizing neuromuscular blocking agents after surgery.
2. DOSAGE AND ADMINISTRATION
2.1. Important Dosage Information
BLOXIVERZ should be administered by trained healthcare providers familiar with the use, actions, characteristics, and complications of neuromuscular blocking agents (NMBA) and neuromuscular block reversal agents. Doses of BLOXIVERZ should be individualized, and a peripheral nerve stimulator should be used to determine the time of initiation of BLOXIVERZ and should be used to determine the need for additional doses.
BLOXIVERZ is for intravenous use only and should be injected slowly over a period of at least 1 minute. The BLOXIVERZ dosage is weight-based [see Dosage and Administration (2.2)].
Prior to BLOXIVERZ administration and until complete recovery of normal ventilation, the patient should be well ventilated and a patent airway maintained. Satisfactory recovery should be judged by adequacy of skeletal muscle tone and respiratory measurements in addition to the response to peripheral nerve stimulation.
An anticholinergic agent, e.g., atropine sulfate or glycopyrrolate, should be administered prior to or concomitantly with BLOXIVERZ [see Dosage and Administration (2.4)]
2.2. Dosage in Adults
- Peripheral nerve stimulation devices capable of delivering a train-of-four (TOF) stimulus are essential to effectively using BLOXIVERZ.
- There must be a twitch response to the first stimulus in the TOF of at least 10% of its baseline level, i.e., the response prior to NMBA administration, prior to the administration of BLOXIVERZ.
- Prior to administration, visually inspect BLOXIVERZ for particulate matter and discoloration.
- BLOXIVERZ should be injected slowly by intravenous route over a period of at least 1 minute.
- A 0.03 mg/kg to 0.07 mg/kg dose of BLOXIVERZ will generally achieve a TOF twitch ratio of 90% (TOF0.9) within 10 to 20 minutes of administration. Dose selection should be based on the extent of spontaneous recovery that has occurred at the time of administration, the half-life of the NMBA being reversed, and whether there is a need to rapidly reverse the NMBA.
- The 0.03 mg/kg dose is recommended for:
-
i. Reversal of NMBAs with shorter half-lives, e.g., rocuronium, or -
ii. When the first twitch response to the TOF stimulus is substantially greater than 10% of baseline or when a second twitch is present.
-
- The 0.07 mg/kg dose is recommended for
-
iii. NMBAs with longer half-lives, e.g., vecuronium and pancuronium, or -
iv. When the first twitch response is relatively weak, i.e., not substantially greater than 10% of baseline or -
v. There is need for more rapid recovery.
-
- The 0.03 mg/kg dose is recommended for:
- TOF monitoring should continue to be used to evaluate the extent of recovery of neuromuscular function and the possible need for an additional dose of BLOXIVERZ.
- TOF monitoring alone should not be relied upon to determine the adequacy of reversal of neuromuscular blockade as related to a patient’s ability to adequately ventilate and maintain a patent airway following tracheal extubation.
- Patients should continue to be monitored for adequacy of reversal from NMBAs for a period of time that would assure full recovery based on the patient’s medical condition and the pharmacokinetics of neostigmine and the NMBA used.
- The recommended maximum total dose is 0.07 mg/kg or up to a total of 5 mg, whichever is less.
2.3. Dosage in Pediatric Patients, including Neonates
Adult guidelines should be followed when BLOXIVERZ is administered to pediatric patients. Pediatric patients require BLOXIVERZ doses similar to those for adult patients.
2.4. Anticholinergic (Atropine or Glycopyrrolate) Administration
An anticholinergic agent, e.g., atropine sulfate or glycopyrrolate, should be administered prior to or concomitantly with BLOXIVERZ. The anticholinergic agent should be administered intravenously using a separate syringe. In the presence of bradycardia, it is recommended that the anticholinergic agent be administered prior to BLOXIVERZ.
3. DOSAGE FORMS AND STRENGTHS
BLOXIVERZ is available as
- Injection: 0.5 mg/mL, 5 mg of neostigmine methylsulfate in 10 mL multiple- dose vials
- Injection: 1 mg/mL, 10 mg of neostigmine methylsulfate in 10 mL multiple-dose vials
4. CONTRAINDICATIONS
BLOXIVERZ is contraindicated in patients with:
- known hypersensitivity to neostigmine methylsulfate (known hypersensitivity reactions have included urticaria, angioedema, erythema multiforme, generalized rash, facial swelling, peripheral edema, pyrexia, flushing, hypotension, bronchospasm, bradycardia and anaphylaxis).
- with peritonitis or mechanical obstruction of the intestinal or urinary tract.
5. WARNINGS AND PRECAUTIONS
5.1. Bradycardia
Neostigmine has been associated with bradycardia. Atropine sulfate or glycopyrrolate should be administered prior to BLOXIVERZ to lessen the risk of bradycardia [see Dosage and Administration (2.4)].
5.2. Serious Side Effects in Patients with Certain Coexisting Conditions
BLOXIVERZ should be used with caution in patients with coronary artery disease, cardiac arrhythmias, recent acute coronary syndrome or myasthenia gravis. Because of the known pharmacology of neostigmine methylsulfate as an acetylcholinesterase inhibitor, cardiovascular effects such as bradycardia, hypotension or dysrhythmia would be anticipated. In patients with certain cardiovascular conditions such as coronary artery disease, cardiac arrhythmias or recent acute coronary syndrome, the risk of blood pressure and heart rate complications may be increased. Risk of these complications may also be increased in patients with myasthenia gravis. Standard antagonism with anticholinergics (e.g., atropine) is generally successful to mitigate the risk of cardiovascular complications.
5.3. Hypersensitivity
Because of the possibility of hypersensitivity, atropine and medications to treat anaphylaxis should be readily available.
5.4. Neuromuscular Dysfunction
Large doses of BLOXIVERZ administered when neuromuscular blockade is minimal can produce neuromuscular dysfunction. The dose of BLOXIVERZ should be reduced if recovery from neuromuscular blockade is nearly complete.
5.5. Cholinergic Crisis
It is important to differentiate between myasthenic crisis and cholinergic crisis caused by overdosage of BLOXIVERZ. Both conditions result in extreme muscle weakness but require radically different treatment. [see Overdosage (10)]
6. ADVERSE REACTIONS
6.1. Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions to neostigmine methylsulfate are most often attributable to exaggerated pharmacological effects, in particular, at muscarinic receptor sites. The use of an anticholinergic agent, e.g., atropine sulfate or glycopyrrolate, may prevent or mitigate these reactions.
Quantitative adverse event data are available from trials of neostigmine methylsulfate in which 200 adult patients were exposed to the product. The following table lists the adverse reactions that occurred with an overall frequency of 1% or greater.
| System Organ Class | Adverse Reaction |
| Cardiovascular Disorders | bradycardia, hypotension, tachycardia/heart rate increase |
| Gastrointestinal Disorders | dry mouth, nausea, post-procedural nausea, vomiting |
|
General Disorders and Administration Site Conditions |
incision site complication, pharyngolaryngeal pain, procedural complication, procedural pain |
| Nervous System Disorders | dizziness, headache, postoperative shivering, prolonged neuromuscular blockade |
| Psychiatric Disorders | insomnia |
|
Respiratory, Thoracic and Mediastinal Disorders |
dyspnea, oxygen desaturation <90% |
| Skin and Subcutaneous Tissue Disorders | pruritus |
6.2. Post Marketing Experience
The following adverse reactions have been identified during parenteral use of neostigmine methylsulfate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
| System Organ Class | Adverse Reaction |
| Allergic Disorders | allergic reactions, anaphylaxis |
| Nervous System Disorders | convulsions, drowsiness, dysarthria, fasciculation, loss of consciousness, miosis, visual changes |
| Cardiovascular Disorders | cardiac arrest, cardiac arrhythmias (A-V block, nodal rhythm), hypotension, nonspecific EKG changes, syncope |
|
Respiratory, Thoracic and Mediastinal Disorders |
bronchospasm; increased oral, pharyngeal and bronchial secretions; respiratory arrest; respiratory depression |
| Skin and Sub-cutaneous Tissue Disorders | rash, urticaria |
| Gastrointestinal Disorders | bowel cramps, diarrhea, flatulence, increased peristalsis |
| Renal and Urinary Disorders | urinary frequency |
|
Musculoskeletal and Connective Tissue Disorders |
arthralgia, muscle cramps, spasms, weakness |
| Miscellaneous | diaphoresis, flushing |
7. DRUG INTERACTIONS
The pharmacokinetic interaction between neostigmine methylsulfate and other drugs has not been studied. Neostigmine methylsulfate is metabolized by microsomal enzymes in the liver. Use with caution when using BLOXIVERZ with other drugs which may alter the activity of metabolizing enzymes or transporters.
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Teratogenic Effects: Pregnancy Category C. It is not known whether neostigmine methylsulfate can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. BLOXIVERZ should be given to a pregnant woman only if clearly needed.
Animal reproduction studies have not been conducted with neostigmine methylsulfate.
8.2. Labor and Delivery
The effect of BLOXIVERZ on the mother and fetus with regard to labor, delivery, the need for forceps delivery or other intervention or resuscitation of the newborn, is not known.
Cholinesterase inhibitor drugs may induce premature labor when given intravenously to pregnant women near term.
8.3. Nursing Mothers
It is not known whether neostigmine methylsulfate is excreted in human milk. Caution should be exercised when BLOXIVERZ is administered to a nursing woman.
8.4. Pediatric Use
BLOXIVERZ is approved for the reversal of the effects of non-depolarizing neuromuscular blocking agents after surgery in pediatric patients of all ages.
Recovery of neuromuscular activity occurs more rapidly with smaller doses of cholinesterase inhibitors in infants and children than in adults. However, infants and small children may be at greater risk of complications from incomplete reversal of neuromuscular blockade due to decreased respiratory reserve. The risks associated with incomplete reversal outweigh any risk from giving higher doses of BLOXIVERZ (up to 0.07 mg/kg or up to a total of 5 mg, whichever is lower).
The dose of BLOXIVERZ required to reverse neuromuscular blockade in children varies between 0.03 mg - 0.07 mg/kg, the same dose range shown to be effective in adults, and should be selected using the same criteria as used for adult patients. [see Clinical Pharmacology (12.3)]
Since the blood pressure in pediatric patients, particularly infants and neonates, is sensitive to changes in heart rate, the effects of an anticholinergic agent (e.g., atropine) should be observed prior to administration of neostigmine to lessen the probability of bradycardia and hypotension.
8.5. Geriatric Use
Because elderly patients are more likely to have decreased renal function, BLOXIVERZ should be used with caution and monitored for a longer period in elderly patients. The duration of action of neostigmine methylsulfate is prolonged in the elderly; however, elderly patients also experience slower spontaneous recovery from neuromuscular blocking agents. Therefore, dosage adjustments are not generally needed in geriatric patients; however, they should be monitored for longer periods than younger adults to assure additional doses of BLOXIVERZ are not required. The duration of monitoring should be predicated on the anticipated duration of action for the NMBA used on the patient. [see Dosage and Administration (2.3)].
8.6. Renal Impairment
Elimination half-life of neostigmine methylsulfate was prolonged in anephric patients compared to normal subjects.
Although no adjustments to BLOXIVERZ dosing appear to be warranted in patients with impaired renal function, they should be closely monitored to assure the effects of the neuromuscular blocking agent, particularly one cleared by the kidneys, do not persist beyond those of BLOXIVERZ. In this regard, the interval for re-dosing the neuromuscular blocking agent during the surgical procedure may be useful in determining whether, and to what extent, post-operative monitoring needs to be extended.
8.7. Hepatic Impairment
The pharmacokinetics of neostigmine methylsulfate in patients with hepatic impairment have not been studied. Neostigmine methylsulfate is metabolized by microsomal enzymes in the liver. No adjustments to the dosing of BLOXIVERZ appear to be warranted in patients with hepatic insufficiency. However, patients should be carefully monitored if hepatically cleared neuromuscular blocking agents were used during their surgical procedure as their duration of action may be prolonged by hepatic insufficiency whereas BLOXIVERZ, which undergoes renal elimination, will not likely be affected. This could result in the effects of the neuromuscular blocking agent outlasting those of BLOXIVERZ. This same situation may arise if the neuromuscular blocking agent has active metabolites. In this regard, the interval for re-dosing the neuromuscular blocking agent during the surgical procedure may be useful in determining whether, and to what extent, post-operative monitoring needs to be extended.
10. OVERDOSAGE
Muscarinic symptoms (nausea, vomiting, diarrhea, sweating, increased bronchial and salivary secretions, and bradycardia) may appear with overdosage of BLOXIVERZ (cholinergic crisis), but may be managed by the use of additional atropine or glycopyrrolate. The possibility of iatrogenic overdose can be lessened by carefully monitoring the muscle twitch response to peripheral nerve stimulation. Should overdosage occur, ventilation should be supported by artificial means until the adequacy of spontaneous respiration is assured, and cardiac function should be monitored.
Overdosage of BLOXIVERZ can cause cholinergic crisis, which is characterized by increasing muscle weakness, and through involvement of the muscles of respiration, may result in death. Myasthenic crisis, due to an increase in the severity of the disease, is also accompanied by extreme muscle weakness and may be difficult to distinguish from cholinergic crisis on a symptomatic basis. However, such differentiation is extremely important because increases in the dose of BLOXIVERZ or other drugs in this class, in the presence of cholinergic crisis or of a refractory or “insensitive” state, could have grave consequences. The two types of crises may be differentiated by the use of edrophonium chloride as well as by clinical judgment.
Treatment of the two conditions differs radically. Whereas the presence of myasthenic crisis requires more intensive anticholinesterase therapy, cholinergic crisis calls for the prompt withdrawal of all drugs of this type. The immediate use of atropine in cholinergic crisis is also recommended. Atropine may also be used to lessen gastrointestinal side effects or other muscarinic reactions; but such use, by masking signs of overdosage, can lead to inadvertent induction of cholinergic crisis.
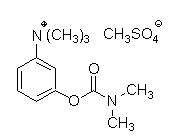
11. DESCRIPTION
Neostigmine methylsulfate, a cholinesterase inhibitor, is (m-hydroxyphenyl) trimethylammonium methylsulfate dimethylcarbamate. The structural formula is:
Neostigmine methylsulfate is a white crystalline powder and is very soluble in water and soluble in alcohol. BLOXIVERZ is a sterile, nonpyrogenic solution intended for intravenous use.
Each mL of the 0.5 mg/mL strength contains neostigmine methylsulfate 0.5 mg, phenol 4.5 mg (used as preservative) and sodium acetate trihydrate 0.2 mg, in water for injection. The pH is adjusted, when necessary, with acetic acid/sodium hydroxide to a value of 5.5.
Each mL of the 1 mg/mL strength contains neostigmine methylsulfate 1 mg, phenol 4.5 mg (used as preservative), and sodium acetate trihydrate 0.2 mg, in water for injection. The pH is adjusted, when necessary, with acetic acid/sodium hydroxide to achieve a value of 5.5.
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
Neostigmine methylsulfate is a competitive cholinesterase inhibitor. By reducing the breakdown of acetylcholine, neostigmine methylsulfate induces an increase in acetylcholine in the synaptic cleft which competes for the same binding site as nondepolarizing neuromuscular blocking agents, and reverses the neuromuscular blockade.
12.2. Pharmacodynamics
Neostigmine methylsulfate-induced increases in acetylcholine levels results in the potentiation of both muscarinic and nicotinic cholinergic activity. The resulting elevation of acetylcholine competes with nondepolarizing neuromuscular blocking agents to reverse neuromuscular blockade. Neostigmine methylsulfate does not readily cross the blood-brain barrier and, therefore, does not significantly affect cholinergic function in the central nervous system.
12.3. Pharmacokinetics
Distribution: Following intravenous injection, the observed neostigmine methylsulfate volume of distribution is reported between 0.12 and 1.4 L/kg. Protein binding of neostigmine methylsulfate to human serum albumin ranges from 15 to 25%.
Metabolism: Neostigmine methylsulfate is metabolized by microsomal enzymes in the liver.
Elimination: Following intravenous injection, the reported elimination half-life of neostigmine methylsulfate is between 24 and 113 minutes. Total body clearance of neostigmine methylsulfate is reported between 1.14 and 16.7 mL/min/kg.
Renal impairment: Elimination half-life of neostigmine methylsulfate was prolonged in anephric patients compared to normal subjects; elimination half-life for normal, transplant and anephric patients were 79.8 ± 48.6, 104.7 ± 64 and 181 ± 54 min (mean ± SD), respectively.
Hepatic impairment: The pharmacokinetics of neostigmine methylsulfate in patients with hepatic impairment has not been studied.
Pediatrics: Elimination half-life of neostigmine methylsulfate in infants (2-10 months), children (1-6 years) and adults (29-48 years) were 39 ± 5 min, 48 ± 16 min, and 67 ± 8 min (mean ± SD), respectively. Observed neostigmine methylsulfate clearance for infants, children and adults were 14 ± 3, 11 ± 3 and 10 ± 2 mL/min/kg (mean ± SD), respectively.
Drug Interaction Studies: The pharmacokinetic interaction between neostigmine methylsulfate and other drugs has not been studied.
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Long-term animal studies have not been performed to evaluate the carcinogenic potential of neostigmine.
Mutagenesis: Neostigmine methylsulfate was not mutagenic in an in vitro bacterial reverse mutation assay (Ames test).
Impairment of Fertility: Studies on the effect of neostigmine methylsulfate on fertility have not been performed.
14. CLINICAL STUDIES
The evidence for the efficacy of neostigmine methylsulfate for the reversal of the effects of non-depolarizing neuromuscular blocking agents after surgery is derived from the published literature. Randomized, spontaneous-recovery or placebo-controlled studies using similar efficacy endpoints evaluated a total of 404 adult and 80 pediatric patients undergoing various surgical procedures. Patients had reductions in their recovery time from neuromuscular blockade with neostigmine methylsulfate treatment compared to spontaneous recovery.
16. HOW SUPPLIED/STORAGE AND HANDLING
BLOXIVERZ (Neostigmine Methylsulfate Injection, USP) is available in the following:
| NDC No. | Strength | Vial Size |
| 76014-002-10 | 0.5 mg/mL | 10 mL multiple-dose vials supplied in packages of 10 |
| 76014-002-33 | 0.5 mg/mL | 10 mL multiple-dose vial supplied in single carton |
| 76014-003-10 | 1 mg/mL | 10 mL multiple-dose vials supplied in packages of 10 |
| 76014-003-33 | 1 mg/mL | 10 mL multiple-dose vial supplied in single carton |
The vial stopper is not made with natural rubber latex.
BLOXIVERZ should be stored at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (see USP Controlled Room Temperature). Protect from light. Store in carton until time of use.
Manufactured for:
Éclat Pharmaceuticals
Chesterfield, MO 63005 USA
Package Label - Principal Display Panel - Neostigmine Methylsulfate - 0.5 mg Vial - NDC 76014-002-33

Package Label - Principal Display Panel - Neostigmine Methylsulfate - 0.5 mg Carton - NDC 76014-002-33

Package Label - Principal Display Panel - Neostigmine Methylsulfate - 0.5 mg Ten Carton Package - NDC 76014-002-10

Package Label - Principal Display Panel - Neostigmine Methylsulfate - 1.0 mg Vial - NDC 76014-003-33

Package Label - Principal Display Panel - Neostigmine Methylsulfate - 1.0 mg Carton - NDC 76014-003-33

Package Label - Principal Display Panel - Neostigmine Methylsulfate - 1.0 mg Ten Carton Package - NDC 76014-003-10

BloxiverzNeostigmine Methylsulfate INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
BloxiverzNeostigmine Methylsulfate INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||