Blistex
Blistex Daily Conditioning Treatment
FULL PRESCRIBING INFORMATION: CONTENTS*
- Blistex Uses
- Warnings
- Directions
- Blistex Other information
- Inactive ingredients
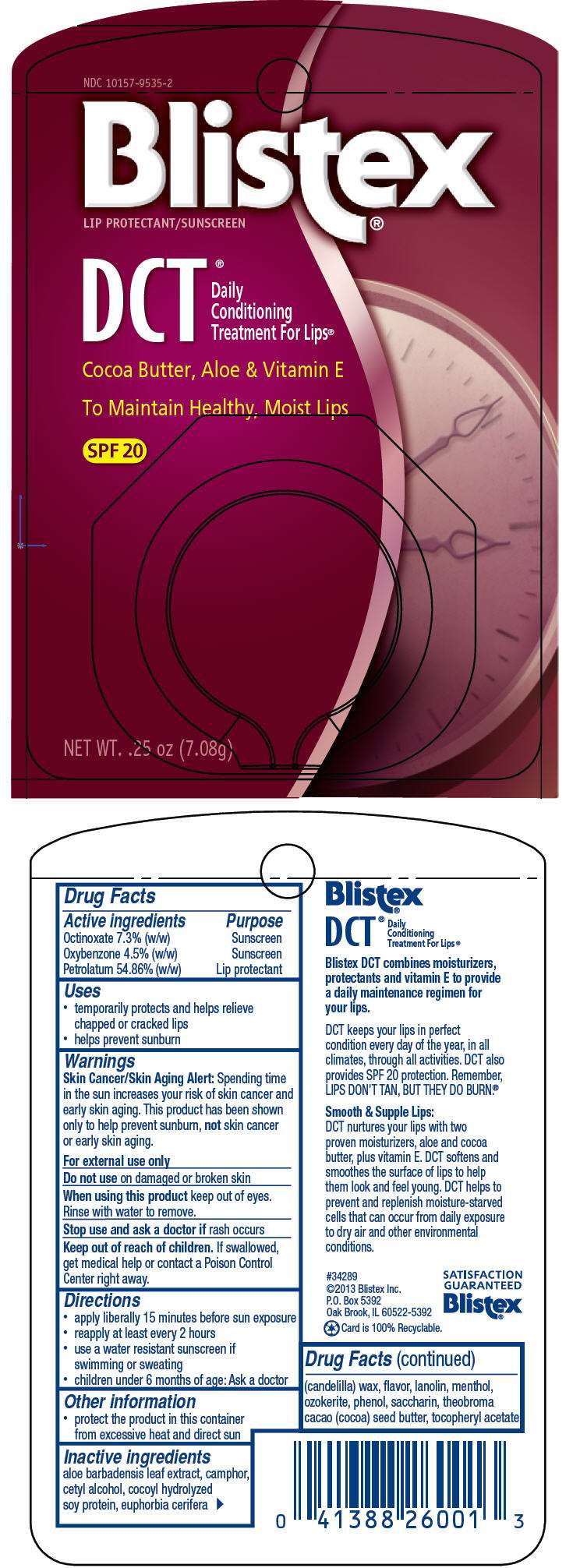
- PRINCIPAL DISPLAY PANEL - 7.08 g Jar Blister Pack
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose |
|---|---|
| Octinoxate 7.3% (w/w) | Sunscreen |
| Oxybenzone 4.5% (w/w) | Sunscreen |
| Petrolatum 54.86% (w/w) | Lip protectant |
Blistex Uses
- temporarily protects and helps relieve chapped or cracked lips
- helps prevent sunburn
Warnings
Skin Cancer/Skin Aging Alert
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- children under 6 months of age: Ask a doctor
Blistex Other information
- protect the product in this container from excessive heat and direct sun
Inactive ingredients
aloe barbadensis leaf extract, camphor, cetyl alcohol, cocoyl hydrolyzed soy protein, euphorbia cerifera (candelilla) wax, flavor, lanolin, menthol, ozokerite, phenol, saccharin, theobroma cacao (cocoa) seed butter, tocopheryl acetate
PRINCIPAL DISPLAY PANEL - 7.08 g Jar Blister Pack
NDC 10157-9535-2
Blistex
®
LIP PROTECTANT/SUNSCREEN
DCT ®
Daily
Conditioning
Treatment For Lips®
Cocoa Butter, Aloe & Vitamin E
To Maintain Healthy, Moist Lips
SPF 20
NET WT. .25 oz (7.08g)
BlistexPetrolatum, Octinoxate, and Oxybenzone PASTE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||