Blistex
Blistex European Formula Lip Conditioner™
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
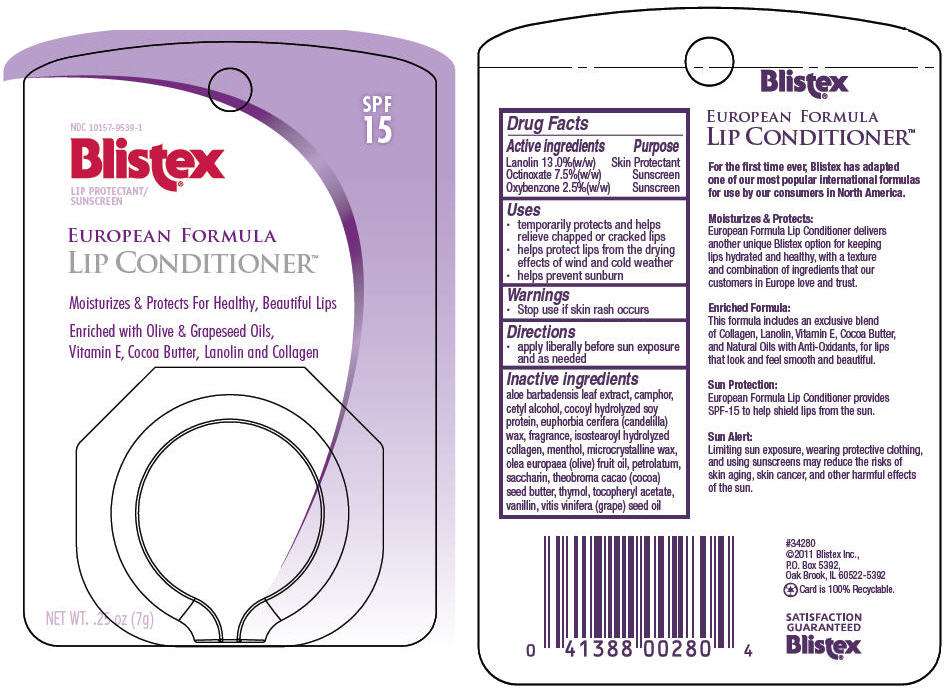
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose |
|---|---|
| Lanolin 13 .0%(w/w) | Skin Protectant |
| Octinoxate 7.5%(w/w) | Sunscreen |
| Oxybenzone 2.5%(w/w) | Sunscreen |
Blistex Uses
- temporarily protects and helps relieve chapped or cracked lips
- helps protect lips from the drying effects of wind and cold weather
- helps prevent sunburn
Warnings
- Stop use if skin rash occurs
Directions
- apply liberally before sun exposure and as needed
Inactive ingredients
aloe barbadensis leaf extract, camphor, cetyl alcohol, cocoyl hydrolyzed soy protein, euphorbia cerifera (candelilla) wax, fragrance, isostearoyl hydrolyzed collagen, menthol, microcrystalline wax, olea europaea (olive) fruit oil, petrolatum, saccharin, theobroma cacao (cocoa) seed butter, thymol, tocopheryl acetate, vanillin, vitis vinifera (grape) seed oil
#34280
©2011 Blistex Inc.,
P.O. Box 5392,
Oak Brook, IL 60522-5392
PRINCIPAL DISPLAY PANEL - 7 g Container Label
NDC 10157-9539-1
Blistex ®
LIP PROTECTANT/
SUNSCREEN
SPF
15
EUROPEAN FORMULA
LIP CONDITIONER™
Moisturizes & Protects For Healthy, Beautiful Lips
Enriched with Olive & Grapeseed Oils,
Vitamin E, Cocoa Butter, Lanolin and Collagen
NET WT. .25 oz (7g)
BlistexLanolin, Octinoxate, and Oxybenzone PASTE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||