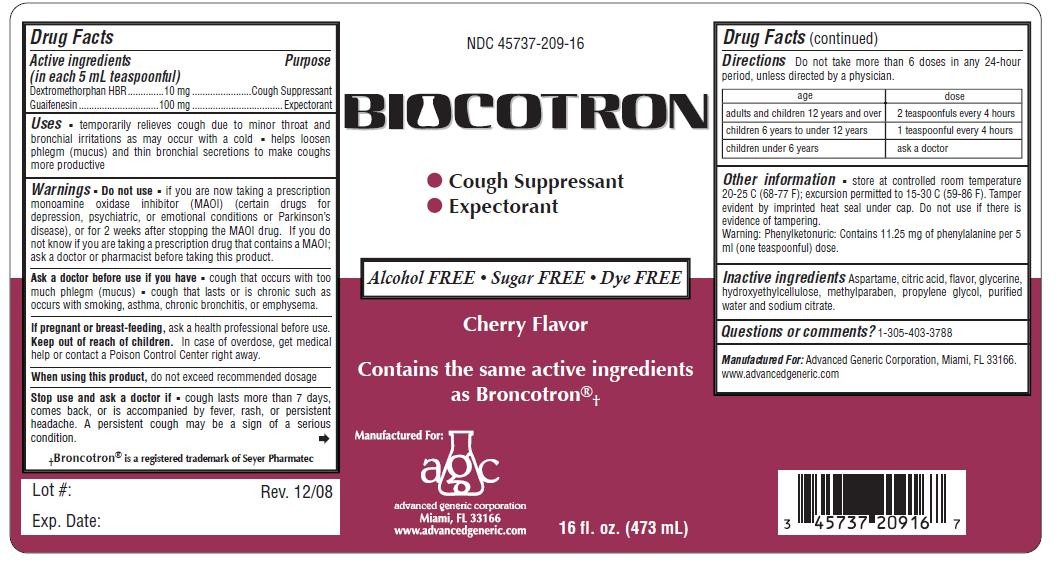
Biocotron
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients: (in each 5 mL tsp.) Purpose
Dextromethorphan Hydrobromide 10 mg.................. Cough Suppressant
Guaifenesin 100 mg .............................................. Expectorant
Purpose
Uses
- temporarily relieves cough due to minor throat and bronchial irritations as may occur with a cold
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema.
When using this product, do not exceed recommended dosage
Do not Use
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Directions Do not take more than 6 doses in any 24-hour period, unless directed by a physician
adults and children 12 years and over, 2 teaspoonfuls every 4 hours
children 6 years to under 12 years, 1 teaspoonful every 4 hours
Uses
Other information store at controlled room temperature
20-25 C (68-77 F); excursion permitted to 15-30 C (59-86 F). Tamper
evident by imprinted heat seal under cap. Do not use if there is
evidence of tampering.
Warning: Phenylketonuric: Contains 11.25 mg of phenylalanine per 5 ml (one teaspoonful) dose.
Inactive ingredients Aspartame, citric acid, flavor, glycerine,
hydroxyethylcellulose, methylparaben, propylene glycol, purified
water and sodium citrate.
Questions or comments? 1-305-403-3788
Manufactured For: Advanced Generic Corporation, Miami, FL 33166.
www.advancedgeneric.com
BiocotronDextromethorphan, Guaifenesin LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||