biclora
Hawthorn Pharmaceuticals, Inc.
Pernix Manufacturing, LLC
biclora
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- biclora Uses
- Warnings
- Directions
- biclora Other information
- Inactive ingredients
- Questions? Comments?
- Product Packaging
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredients(in each tablet)
Purpose
biclora Uses
- cough due to minor throat and bronchial irritation
- calms the cough control center and relieves coughing
- runny nose
- sneezing
- itching of the nose or throat
- itchy, water eyes
Warnings
Ask a doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
- a cough that occurs with too much phlegm (mucus)
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to the enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
When using this product
- excitability may occur, especially in children
- may cause marked drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase the drowsiness effect
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- cough persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or persistent headache. A persistent cough may be a sign of a serious condition.
- new symptoms occur
Keep out of reach of children.
Directions
Do not exceed recommended dosage.| Adults and children 12 years of age and over: |
1 tablet every 6 to 8 hours, not to exceed 3 tablets in 24 hours or as directed by a doctor. |
| Children 6 to under 12 years of age: |
1/2 tablet every 6 to 8 hours, not to exceed 1 1/2 tablets in 24 hours, or as directed by a doctor. |
| Children under 6 years of age: |
Consult a doctor. |
biclora Other information
Inactive ingredients
Questions? Comments?
Product Packaging
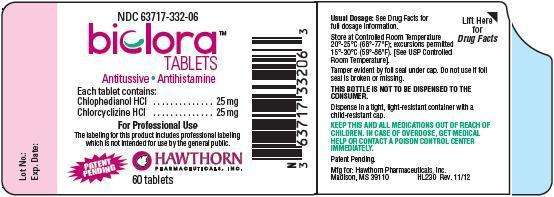
biclora
·
For Professional Use
HAWTHORN
Usual Dosage:
THIS BOTTLE IS NOT TO BE DISPENSED TO THE CONSUMER.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF
OVERDOSE, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER
IMMEDIATELY.

bicloraChlorphedianol Hydrochloride, Chlorcyclizine Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!