BenzePrO
BenzePrO Short Contact Foam9.8% Benzoyl Peroxide aerosol
FULL PRESCRIBING INFORMATION: CONTENTS*
- BENZEPRO DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- BENZEPRO CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- BENZEPRO ADVERSE REACTIONS
- OVERDOSAGE
- INSTRUCTIONS FOR USE (DIRECTIONS)
- HOW SUPPLIED
- NDC 42546-015-10PruGen PharmaceuticalsRx OnlyBenzePrO Short Contact FoamBenzoyl Peroxide 9.8%Net Weight 100gManufactured For:PruGen, Inc. Pharmaceuticals8714 E Vista Bonita DrScottsdale, AZ 85255 Rev 1.1
FULL PRESCRIBING INFORMATION
BENZEPRO DESCRIPTION
Each gram of BenzePrO™ Short Contact Foam contains 9.8% benzoyl peroxide in an aqueous based emollient foam vehicle containing BHT, C12-15 alkyl benzoate, cetostearyl alcohol, citric acid, dimethicone, disodium EDTA, emulsifying wax, glycerin, methylparaben, povidone, propylene glycol, propylparaben, purified water, sodium citrate, steareth-10, stearic acid.
Also contains: Propellant HFA-134A (1,1,1,2-tetrafluoroethane).
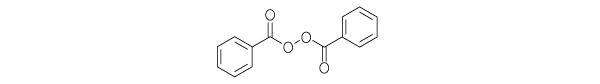
Benzoyl peroxide is an oxidizing agent that possesses antibacterial properties and is classified as a keratolytic. Benzoyl peroxide (C14H10O4) is represented by the following structure:
CLINICAL PHARMACOLOGY
The exact method of action of benzoyl peroxide in acne vulgaris is not known. Benzoyl peroxide is an antibacterial agent with demonstrated activity against Propionibacterium acnes. This action, combined with the mild keratolytic effect of benzoyl peroxide, is believed to be responsible for its usefulness in acne. Benzoyl peroxide is absorbed by the skin where it is metabolized to benzoic acid and excreted as benzoate in the urine.
INDICATIONS & USAGE
BenzePrO™ Short Contact Foam is indicated for use in the topical treatment of mild to moderate acne vulgaris.
BENZEPRO CONTRAINDICATIONS
BenzePrO™ Short Contact Foam should not be used in patients who have shown hypersensitivity to benzoyl peroxide or to any of the other ingredients in the product. Discontinue use if hypersensitivity is observed.
WARNINGS
FOR EXTERNAL USE ONLY. Not For Ophthalmic Use. Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
When using this product, skin irritation and dryness is more likely to occur if:
• you leave BenzePrO™ Short Contact Foam on your skin longer than directed
• you use another topical acne medication at the same time
Do not use this product if you:
• have very sensitive skin
• are sensitive to benzoyl peroxide
When using this product:
• avoid unnecessary sun exposure and use a sunscreen
• avoid contact with the eyes, lips, mouth
• avoid contact with hair and dyed fabrics, which may be bleached by this product
• skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the
product less frequently or in lower concentration.
Stop use and ask a doctor:
• if irritation becomes severe.
Contents under pressure. Do not puncture or incinerate container. Do not expose to temperatures above 120°F (49°C).
PRECAUTIONS
General:
If severe irritation develops, discontinue use and institute appropriate therapy.
Information for patients:
This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. Avoid contact with eyes, eyelids, lips, and mucous membranes. If accidental contact occurs, rinse with water. If excessive redness or irritation develops, discontinue use and consult your physician.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Based upon all available evidence, benzoyl peroxide is not considered to be a carcinogen. However, data from a study using mice known to be highly susceptible to cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of the findings is not known.
Pregnancy:
Category C animal reproduction studies have not been conducted with benzoyl peroxide. It is also not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzoyl peroxide should be used by a pregnant woman only if clearly needed.
Nursing Mothers:
It is not known whether this drug is excreted in the human milk. Because many drugs are excreted in human milk, caution should be exercised when benzoyl peroxide is administered to a nursing woman.
Pediatric Use:
Safety and effectiveness in children below the age of 12 have not been established.
BENZEPRO ADVERSE REACTIONS
Allergic contact dermatitis and dryness have been reported with topical benzoyl peroxide therapy.
OVERDOSAGE
If excessive scaling, erythema or edema occurs, the use of this preparation should be discontinued. To hasten resolution of the adverse effects, cool compresses may be used. After symptoms and signs subside, a reduced dosage schedule may be cautiously tried if the reaction is judged to be due to excessive use and not allergenicity.
INSTRUCTIONS FOR USE (DIRECTIONS)
Avoid contact with hair, fabrics or carpeting as benzoyl peroxide will cause bleaching or discoloration.
Prime can before initial use:
Shake can vigorously (until product moves inside can). Firmly strike bottom of can onto palm of other hand or a hard surface at least 3 times. Hold can upright over sink. Direct initial spray to a non-skin surface such as into cap of can. Press down on actuator for 1-3 seconds until foam begins to dispense. If foam does not dispense within 3 seconds, prime can again.
Before Each Use:
Shake vigorously and gently tap bottom of can onto palm of other hand or a solid surface at least 3 times.
During Use:
Holding can upright, dispense BenzePrO™ Short Contact Foam into palm of hand. Cover the entire affected area with a thin layer 1 to 3 times daily. Rub gently over the affected area until foam disappears. Rinse off after 2 minutes. Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two to three times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day. Wash hands with soap and water after use.
• If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
HOW SUPPLIED
BenzePrO™ Short Contact Foam is supplied in a 100g (NDC 42546-015-10) canister.
Will not dispense entire contents. Container is overfilled to guarantee dispensing at least the listed amount.
Store at room temperature: 59° - 77°F (15° - 25°C). Protect from freezing. Store upright.
NDC 42546-015-10PruGen PharmaceuticalsRx OnlyBenzePrO Short Contact FoamBenzoyl Peroxide 9.8%Net Weight 100gManufactured For:PruGen, Inc. Pharmaceuticals8714 E Vista Bonita DrScottsdale, AZ 85255 Rev 1.1

BenzePrOBenzoyl Peroxide AEROSOL, FOAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||