BenzEFoam Ultra
Onset Dermatologics LLC
Onset Dermatologics LLC
FULL PRESCRIBING INFORMATION: CONTENTS*
- BENZEFOAM ULTRA DESCRIPTION
- CLINICAL PHARMACOLOGY
- BENZEFOAM ULTRA INDICATIONS AND USAGE
- BENZEFOAM ULTRA CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- BENZEFOAM ULTRA ADVERSE REACTIONS
- OVERDOSAGE
- BENZEFOAM ULTRA DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
BENZEFOAM ULTRA DESCRIPTION
Each gram of BenzEFoamUltraTM Short Contact Foam contains 9.8% benzoyl peroxide in an aqueous based emollient foam vehicle containing BHT, C12-15 alkyl benzoate, cetearyl alcohol, citric acid, dimethicone, disodium EDTA, emulsifying wax, glycerin, methylparaben, propylene glycol, propylparaben, purified water, sodium citrate, steareth10. Also contains: Propellant HFA-134a (1,1,1,2-tetrafluoroethane).
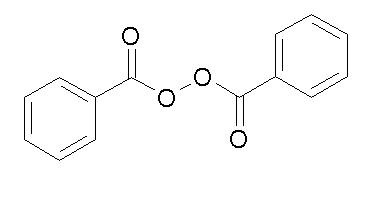
Benzoyl peroxide is an oxidizing agent that possesses antibacterial properties and is classified as a keratolytic. Benzoyl peroxide (C14H10O4) is represented by the following structure:
CLINICAL PHARMACOLOGY
The exact method of action of benzoyl peroxide in acne vulgaris is not known. Benzoyl peroxide is an antibacterial agent with demonstrated activity against Propionibacterium acnes . This action, combined with the mild keratolytic effect of benzoyl peroxide, is believed to be responsible for its usefulness in acne. Benzoyl peroxide is absorbed by the skin where it is metabolized to benzoic acid and excreted as benzoate in the urine.
BENZEFOAM ULTRA INDICATIONS AND USAGE
BenzEFoamUltraTM Short Contact Foam is indicated for use in the topical treatment of mild to moderate acne vulgaris.
BENZEFOAM ULTRA CONTRAINDICATIONS
BenzEFoamUltraTM Short Contact Foam should not be used in patients who have shown hypersensitivity to benzoyl peroxide or to any of the other ingredients in the product. Discontinue use if hypersensitivity is observed.
WARNINGS
FOR EXTERNAL USE ONLY. Not For Ophthalmic use. Keep out of the reach of children.
When using this product, skin irritation and dryness is more likely to occur if:
- you leave BenzEFoamUltraTM Short Contact Foam on your skin longer than directed
- you use another topical acne medication at the same time
- use BenzEFoamUltraTM Short Contact Foam less frequently
- use one topical acne medication at a time
- stop use and ask a doctor if irritation becomes severe
- have very sensitive skin
- are sensitive to benzoyl peroxide
When using this product :
• avoid unnecessary sun exposure and use a sunscreen
• avoid contact with the eyes, lips, and mouth
• avoid contact with hair and dyed fabrics, which may be bleached by this product
• skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling
Contents under pressure. Do not puncture or incinerate container. Do not expose to temperature above 120°F (49°C).
PRECAUTIONS
General: If severe irritation develops, discontinue use and institute appropriate therapy.
Information for Patients: This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. Avoid contact with eyes, eyelids, lips, and mucous membranes. If accidental contact occurs, rinse with water. If excessive redness or irritation develops, discontinue use and consult your physician.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Based upon all available evidence, benzoyl peroxide is not considered to be a carcinogen. However, data from a study using mice known to be highly susceptible to cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of the findings is not known.
Pregnancy: Category C. Animal reproduction studies have not been conducted with benzoyl peroxide. It is also not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzoyl peroxide should be used by a pregnant woman only if clearly needed.
Nursing Mothers: It is not known whether this drug is excreted in the human milk. Because many drugs are excreted in human milk, caution should be exercised when benzoyl peroxide is administered to a nursing woman.
Pediatric Use: Safety and effectiveness in children below the age of 12 have not been established.
BENZEFOAM ULTRA ADVERSE REACTIONS
Allergic contact dermatitis and dryness have been reported with topical benzoyl peroxide therapy.
OVERDOSAGE
If excessive scaling, erythema or edema occurs, the use of this preparation should be discontinued. To hasten resolution of the adverse effects, cool compresses may be used. After symptoms and signs subside, a reduced dosage schedule may be cautiously tried if the reaction is judged to be due to excessive use and not allergenicity.
BENZEFOAM ULTRA DOSAGE AND ADMINISTRATION
- Avoid contact with hair, fabrics or carpeting as benzoyl peroxide may cause bleaching or discoloration.
Prime Can Before Initial Use: Gently push up on actuator with thumb until tab breaks. Shake can vigorously (until product moves inside can). Firmly strike bottom of can onto palm of other hand or a hard surface at least 3 times. Holding can upright over sink, direct initial spray to a non-skin surface. Until primed, DO NOT spray directly on the skin as the initial spray may expel cold liquid propellant. Press down on actuator for 1-3 seconds until foam begins to dispense. If foam does not dispense within 3 seconds, prime can again.
Before Each Use: Shake can vigorously. Firmly strike bottom of can onto palm of other hand or a hard surface at least 3 times.
During Use: Holding can upright, dispense BenzEFoamUltraTM into palm of hand. Cover the entire affected area with a thin layer 1 to 3 times daily. Rub in until completely absorbed. Rinse off after 2 minutes. Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two to three times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day. Wash hands with soap and water after use. If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
HOW SUPPLIED
BenzEFoamUltraTM Short Contact Foam is supplied in a 100g (NDC 16781-201-96) aluminum cans. Will not dispense entire contents. Container is overfilled to guarantee dispensing at least the listed amount.
Store at room temperature: 59° - 77°F (15° - 25°C).
Protect from freezing.
Store upright.
Manufactured in USA For:

Onset Therapeutics
Cumberland, RI 02864
(888) 713-8154
www.onsettx.com
Patent Pending
P/N 2620 Rev. 0
PATIENT INFORMATION
|
IMPORTANT: For use on skin only (topical use). When using this product, avoid unnecessary sun exposure and use a sunscreen. Avoid contact with hair and dyed fabrics, which may be bleached by this product. When using this product, skin irritation may occur. Stop use and ask a doctor if irritation becomes severe. |
Read the Patient Information that comes with BenzEFoamUltraTM Short Contact Foam before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of speaking with your doctor about your medical condition or your treatment.
What is BenzEFoamUltraTM Short Contact Foam ?
BenzEFoamUltraTM Short Contact Foam is a prescription medicine used on the skin (topical) to treat mild to moderate acne vulgaris.
Who should not use BenzEFoamUltraTM Short Contact Foam?Do not use BenzEFoamUltraTM Short Contact Foam if you have very sensitive skin or are sensitive to benzoyl peroxide.
What should I tell my doctor before using BenzEFoamUltraTM Short Contact Foam?Before using BenzEFoamUltraTM Short Contact Foam, tell your doctor about all of your medical conditions, including if you:
- have any allergies.
- are pregnant or planning to become pregnant. It is not known if BenzEFoamUltraTM Short Contact Foam will harm your unborn baby.
- are breastfeeding or plan to breast-feed. It is not known if BenzEFoamUltraTM Short Contact Foam passes into your breast milk.
Tell your doctor about all the medicines and skin products you use. Other skin and topical acne products may increase the irritation of your skin when used with BenzEFoamUltraTM Short Contact Foam. Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
Instructions for applying BenzEFoamUltraTM Short Contact Foam
CAUTION: BenzEFoamUltraTM Short Contact Foam may bleach hair or colored fabric .
- Prime Can Before Initial Use: Gently push up on actuator with thumb until tab breaks. Shake can vigorously (until product moves inside can). Firmly strike bottom of can onto palm of other hand or a hard surface at least 3 times. Holding can upright over sink, direct initial spray to a nonskin surface. Until primed, DO NOT spray directly on the skin as the initial spray may expel cold liquid propellant. Press down on actuator for 1-3 seconds until foam begins to dispense. If foam does not dispense within 3 seconds, prime can again.
- Before Each Use: Shake can vigorously. Firmly strike bottom of can onto palm of other hand or a hard surface at least 3 times.
- During Use: Holding can upright, dispense BenzEFoamUltraTM into palm of hand. Cover the entire affected area with a thin layer 1 to 3 times daily. Rub in until completely absorbed. Rinse off after 2 minutes. Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two to three times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day. Wash hands with soap and water after use. If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.

- Will not dispense entire contents. Container is overfilled to guarantee dispensing at least the listed amount.
What should I avoid while using BenzEFoamUltraTM Short Contact Foam?
- Limit your time in sunlight. Avoid using tanning beds or sun lamps. If you have to be in sunlight, wear a wide-brimmed hat or other protective clothing, and a sunscreen with SPF 15 rating or higher. Your doctor can give you more information about why this is important.
- Do not wash the affected area more than 2 to 3 times a day. Washing or scrubbing your skin too often may make your acne worse.
What are the possible side effects with BenzEFoamUltraTM Short Contact Foam?
Side effects with BenzEFoamUltraTM Short Contact Foam include:
- Skin irritation. Skin irritation and dryness is more likely to occur if you leave BenzEFoamUltraTM on your skin longer than directed or use another topical acne medication at the same time. Stop use and ask a doctor if irritation becomes severe.
This is not the only possible side effect with BenzEFoamUltraTM Short Contact Foam. Call your doctor for medical advice about side effects.
You may report side effects to Onset Therapeutics at 1-888-713-8154.
How should I store BenzEFoamUltraTM Short Contact Foam?
Store at room temperature: 59°-77°F (15°-25°C). Do not expose to temperatures above 120°F (49°C). Protect from freezing. Store upright.
Keep BenzEFoamUltraTM Short Contact Foam and all medicines out of the reach of children.General information about BenzEFoamUltraTM Short Contact Foam
Medicines are sometimes prescribed for conditions that are not mentioned in Patient Information leaflets. Do not use BenzEFoamUltraTM for a condition for which it was not prescribed. Do not give BenzEFoamUltraTM Emollient Foam to other people, even if they have the same condition you have. This leaflet summarizes the most important information about BenzEFoamUltraTM Emollient Foam. If you would like more information, talk with your doctor. You can also ask your doctor or pharmacist for information about BenzEFoamUltraTM Emollient Foam that is written for healthcare professionals. For more information about BenzEFoamUltraTM Emollient Foam, call 1-888-713-8154.
What are the ingredients in BenzEFoamUltraTM Short Contact Foam?
Active Ingredient: benzoyl peroxide 9.8%
Inactive Ingredients: BHT, C12-15 alkyl benzoate, cetearyl alcohol, citric acid, dimethicone, disodium EDTA, emulsifying wax, glycerin, methylparaben, propylene glycol, propylparaben, purified water, sodium citrate, steareth-10. Also contains: Propellant HFA-134a (1,1,1,2-tetrafluoroethane).
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - BenzEFoamUltraTM 5 Grams Carton Label
NDC 16781-201-06
Rx Only
BenzEFoamTM Ultra
Short Contact Foam
benzoyl peroxide 9.8%
For topical treatment of mild to moderate acne vulgaris
Onset THERAPEUTICS
Professional Samples
Enclosed: Six 5g Samples
Available in 100g Cans

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - BenzEFoamUltraTM 5 Grams Can Label
NDC 16781-201-06
Rx Only
Professional Sample
Not for Sale
Net Weight 5g
BenzEFoamTM Ultra
Short contact Foam
benzoyl peroxide 9.8%
Will not dispense entire contents. Container is overfilled to guarantee dispensing a minimum of 5 grams.
BenzEFoam UltraBENZOYL PEROXIDE AEROSOL, FOAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||