BEAUTE INITIALE
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT(S)
AVOBENZONE 2%
OCTINOXATE 7.5%
WARNING
FOR EXTERNAL USE ONLY. NOT TO BE SWALLOWED. AVOID CONTACT WITH EYES. IF CONTACT OCCURS, RINSE EYES THOROUGHLY WITH WATER. DISCONTINUE USE IF SIGNS OF IRRITATION APPEAR. IF IRRITATION PERSISTS, CONSULT A DOCTOR. KEEP OUT OF REACH OF CHILDREN. DO NOT USE ON INFANTS UNDER THE AGE OF SIX MONTHS.
DIRECTIONS FOR USE
APPLY TO THOROUGHLY CLEANSED SKIN, SMOOTHING OVER FACE AND NECK.
INACTIVE INGREDIENTS
AQUA (WATER), ALCOHOL, GLYCERIN, CETEARYL ETHYLHEXANOATE, POLYMETHYLSILSESQUIOXANE, JOJOBA ESTERS, CETYL ALCOHOL, GLYCERYL STEARATE, BUTYROSPERMUM, PARK II (SHEA BUTTER), DIMETHICONE, CYCLOPENTASILOXANE, BUTYLENE GLYCOL, AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER, PEG-75 STEARATE, CETETH-20, STEARETH-20, METHYLPARABEN, PARFUM (FRAGRANCE), PROPYLENE GLYCOL, CAPRYLYL GLYCOL, XANTHAN GUM, AMMONIUM GLYCYRRHIZATE, DISODIUM EDTA, MANGANESE GLUCONATE, POLYMETHYL METHACRYLATE, PEG-12, MAGNESIUM ASPARTATE, ZINC GLUCONATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE, ZINGIBER OFFICINALE (GINGER) ROOT EXTRACT, SILICA, SODIUM CARBOMER, TOCOPHEROL, ASCORBYL TETRAISOPALMITATE, PHENOXYETHANOL, BHT, COPPER GLUCONATE, ETHYLPARABEN, PROPYLPARABEN, SODIUM DNA, CITRIC ACID, CI 17200 (RED 33), CI 77891 (TITANIUM DIOXIDE) MICA
PRINCIPAL DISPLAY PANEL
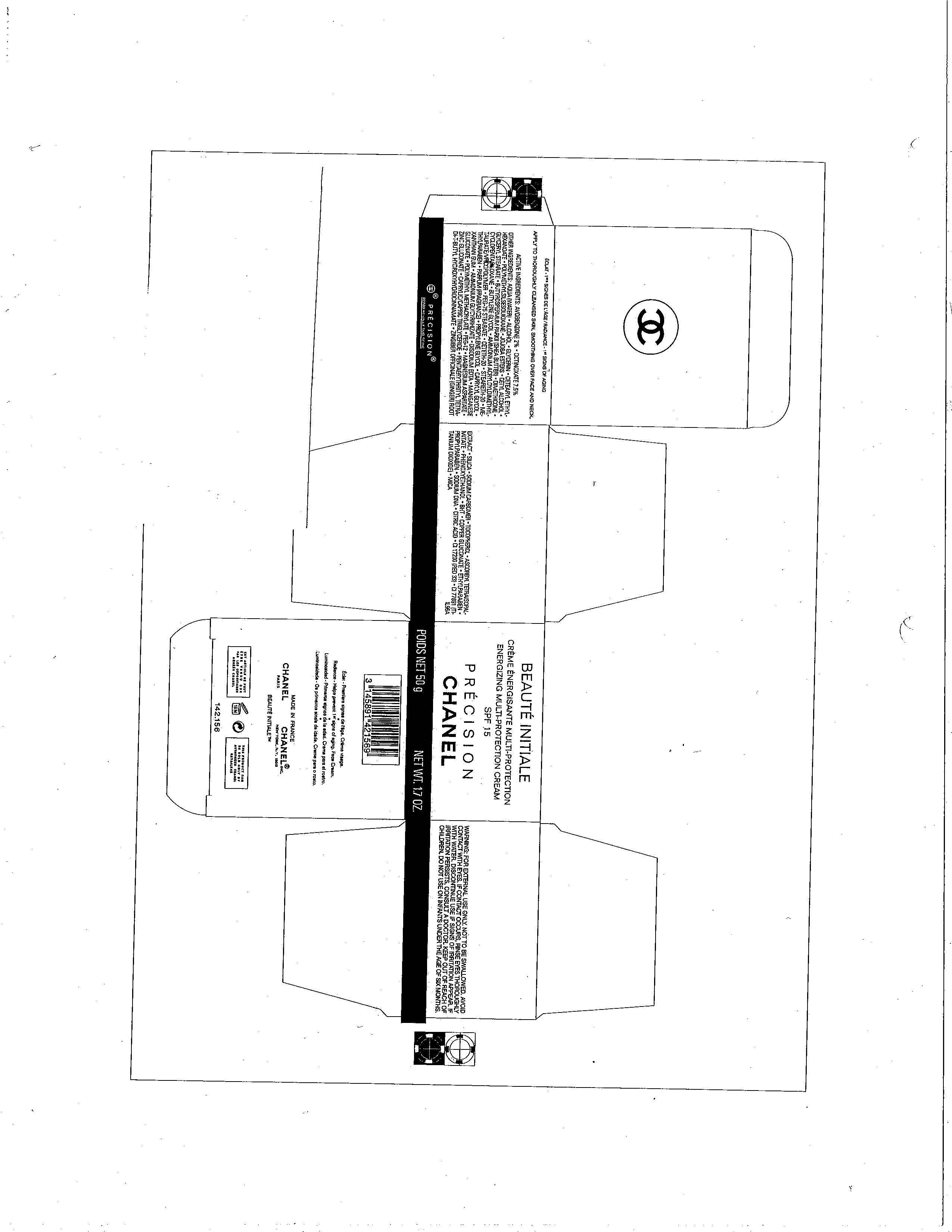
BEAUTE INITIALEENERGIZING MULTI-PROTECTION CREAM CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||