Bay West E2 Antibacterial Skin Cleanser
Wausau Paper Towel & Tissue, LLC
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Bay West E2 Antibacterial Skin Cleanser Uses
- Warnings
- Directions
- Inactive ingredients
- Questions or Comments?
- Package Label - 1200mL
FULL PRESCRIBING INFORMATION
Active ingredient
Chloroxylenol 0.3%
Purpose
Antiseptic handwash
Bay West E2 Antibacterial Skin Cleanser Uses
handwash to help reduce bacteria that potentially can cause disease
Warnings
- For external use only.
Ask a doctor before use if you have
- Deep wounds, animal bites, or serious burns.
When using this product
- Avoid contact with eyes. If this occurs, rinse thoroughly with water.
Stop use and ask a doctor
- If irritation, itching or redness develops. If condition persists for more than 72 hours, consult a doctor.
Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wet hands, apply soap, lather for 30 seconds, and rinse hands thoroughly.
Inactive ingredients
water, sodium lauryl sulfate, sodium chloride, aloe barbadensis leaf juice, propylene glycol, cocamidopropyl betaine, sodium laureth sulfate (and) glycol stearate, cocamide MEA, DMDM hydantoin, methylparaben, fragrance, tocopheryl acetate, triticum vulgare (wheat) germ oil, citric acid.
Questions or Comments?
1-800-723-0001
Package Label - 1200mL
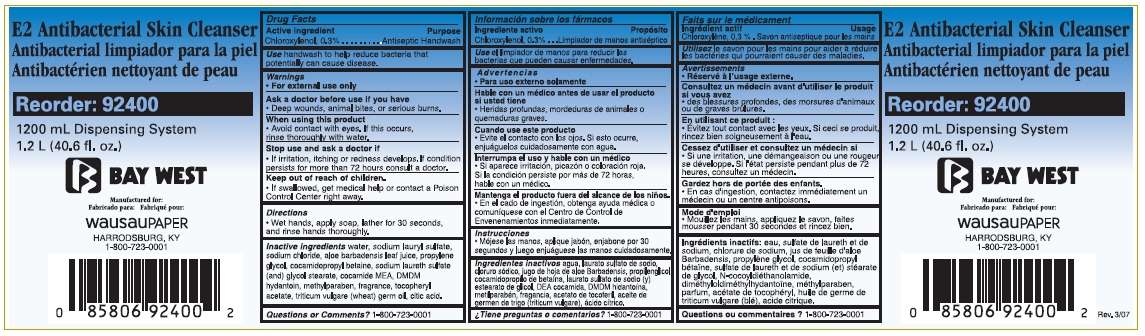
E2 Antibacterial Skin Cleanser
Reorder: 92400
1200 mL Dispensing System
1.2 L (40.6 fl. oz.)
Bay West
Manufactured for:
Wausau Paper
Harrodsburg, KY
1-800-723-0001
Bay West E2 Antibacterial Skin CleanserChloroxylenol SOAP
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!