Banophen
Major Pharmaceuticals Banophen™ Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Banophen Uses
- Warnings
- Directions
- Banophen Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Diphenhydramine hydrochloride 2%
Zinc acetate 0.1%
Purpose
Topical analgesic
Skin protectant
Banophen Uses
- temporarily relieves pain and itching associated with:
- insect bites
- minor burns
- sunburn
- minor skin irritations
- minor cuts
- scrapes
- rashes due to poison ivy, poison oak and poison sumac
- dries the oozing and weeping of poison ivy, poison oak and poison sumac
Warnings
For external use only
Do not use
- on large areas of the body
- with any other product containing diphenhydramine, even one taken by mouth
Ask a doctor before use
- on chicken pox
- on measles
When using this product
- avoid contact with the eyes
Stop use and ask a doctor if
- condition worsens or does not improve within 7 days
- symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- do not use more than directed
- adults and children 2 years of age and older: apply to affected area no more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
Banophen Other information
- store at 20°-25°C (68°-77°F)
Inactive ingredients
cetyl alcohol, diazolidinyl urea, methylparaben, PEG-2 stearate, PEG-20 stearate, propylene glycol, propylparaben, purified water
Questions or comments?
1-800-616-2471
Principal Display Panel
Extra Strength
Banophen™
Anti-Itch Cream
Topical Analgesic/Skin Protectant
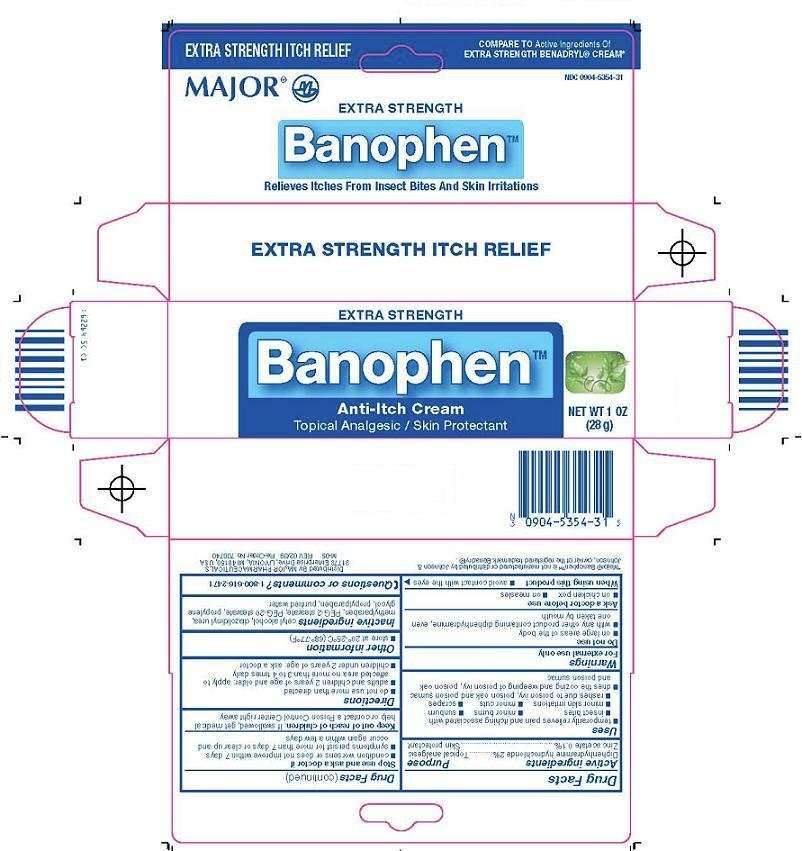
Banophen(tm) Carton
BanophenDiphenhydramine Hydrochloride, Zinc Acetate CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!