Bacitracin
Phoenix Global Supply Group, Inc
Bacitracin Ointment USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (each gram contains)
- Purpose
- Bacitracin Uses
- Warnings
- Directions
- Bacitracin Other information
- Inactive ingredient
- Questions?
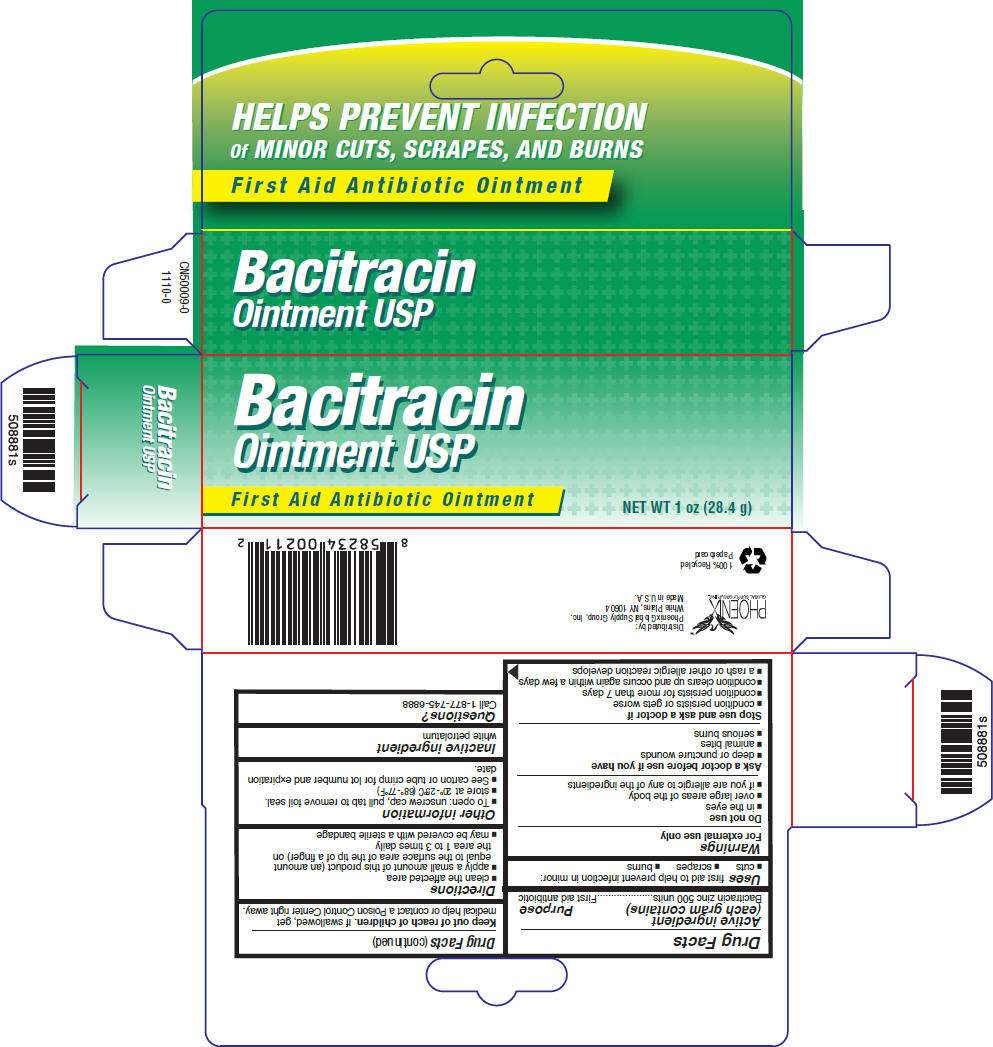
- PRINCIPAL DISPLAY PANEL - 28.4 g Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient (each gram contains)
Bacitracin zinc 500 units
Purpose
First aid antibiotic
Bacitracin Uses
first aid to help prevent infection in minor:
- cuts
- scrapes
- burns
Warnings
For external use only
Do not use
- in the eyes
- over large areas of the body
- if you are allergic to any of the ingredients
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition persists or gets worse
- condition persists for more than 7 days
- condition clears up and occurs again within a few days
- a rash or other allergic reaction develops
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the affected area
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Bacitracin Other information
- To open: unscrew cap, pull tab to remove foil seal.
- store at 20°-25°C (68°-77°F)
- See carton or tube crimp for lot number and expiration date.
Inactive ingredient
white petrolatum
Questions?
Call 1-877-745-6888
PRINCIPAL DISPLAY PANEL - 28.4 g Carton
Bacitracin
Ointment USP
First Aid Antibiotic Ointment
NET WT 1 oz (28.4 g)
BacitracinBacitracin zinc OINTMENT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!