Bacitracin Zinc
Bacitracin Zinc Ointment
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Bacitracin Zinc Uses:
- Ask a doctor before use:
- Bacitracin Zinc Other information
- Inactive ingredients
- Directions
- Stop use and ask a doctor if:
- Keep Out Of Reach Of Children
- Bacitracin Zinc Indications and Usage
- Warnings
FULL PRESCRIBING INFORMATION
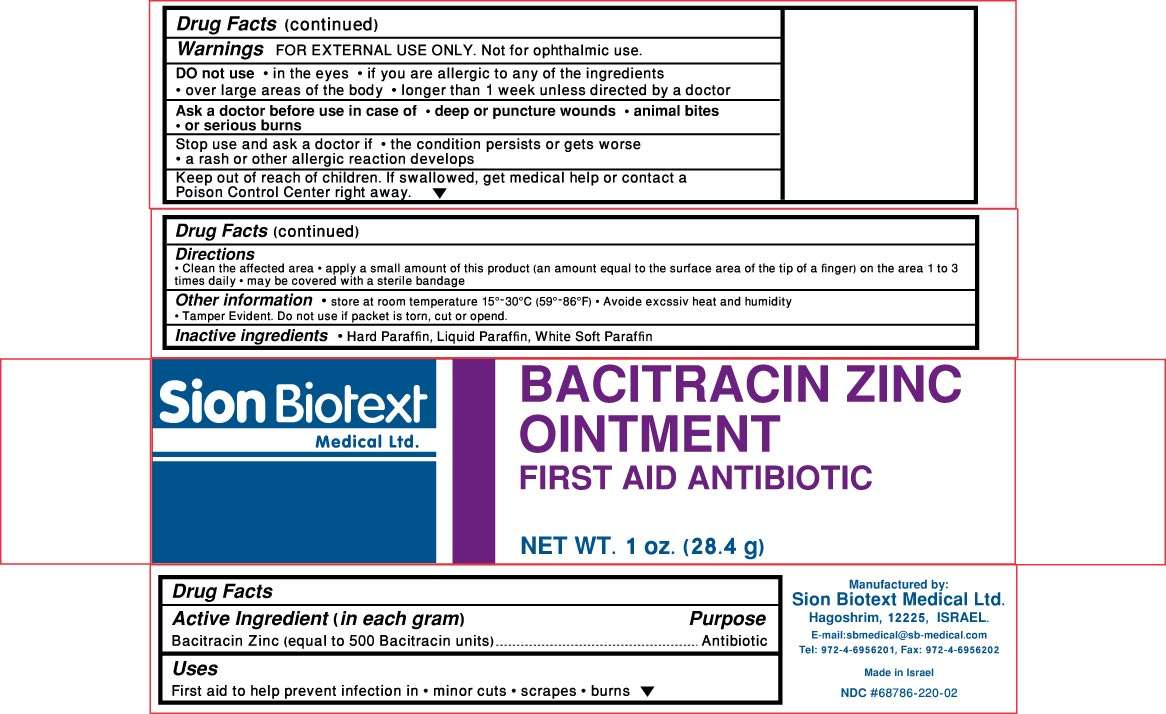
Active Ingredient
Active ingredient Purpose
Bacitracin Zinc (equal to 500 Bacitracin units) Antibiotic
Uses:
- Minor cuts
- scrapes
- burns
Ask a doctor before use:
- in case of deep or puncture wounds
- animal bites
- serious burns
Bacitracin Zinc Other information
- store at controlled room temperature 15°-30° C (59°-86° F)
- Avoid excessive heat and humidity
- tamper evident. Do not use if packet is torn, cut or open.
Inactive ingredients
Hard Paraffin, Liquid Paraffin, White Soft Paraffin
Directions
- clean the affected area
- apply a small amount of this product ( an equal amount to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Stop use and ask a doctor if:
- the condition persists or gets worse
- a rash or other allergic reaction develops
Keep Out Of Reach Of Children
- Keep Out Of Reach Of Children
- If swallowed, get medical help or contact a Poison Control Center right away.
Bacitracin Zinc Indications and Usage
- First aid to help prevent infections
- Not for ophthalmic use
Warnings
- FOR EXTERNAL USE ONLY
- Not for ophthalmic use.
Sion Biotext Medical Ltd
Bacitracin Zinc Ointment
SBM Bacitracin Zinc.jpg
Bacitracin ZincBacitracin Zinc OINTMENT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!